Dual-photon methylglyoxal fluorescent probe and preparation method and application thereof
A methylglyoxal, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of large scattering interference, large probe background interference, low sensitivity and selectivity, etc. Achieve the effect of high yield, convenient purification and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
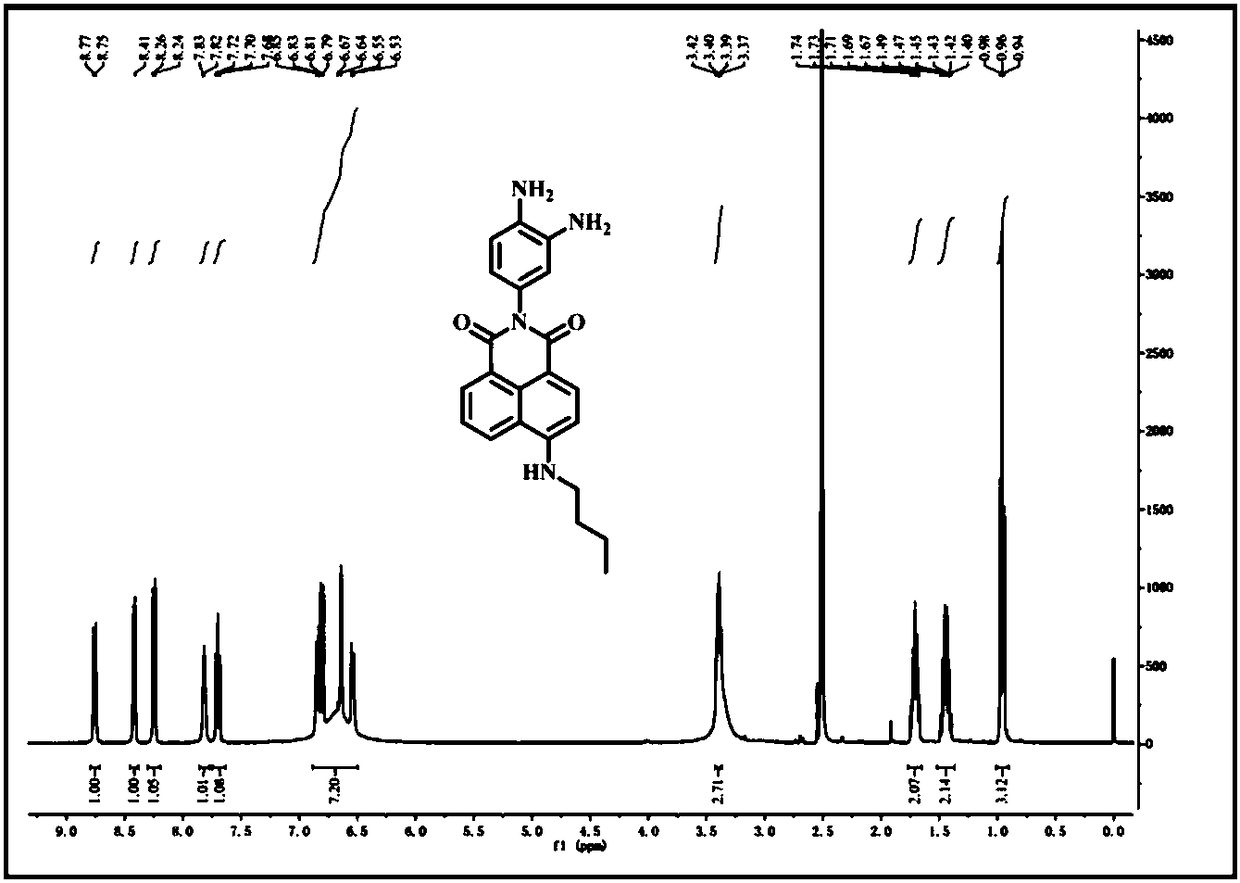
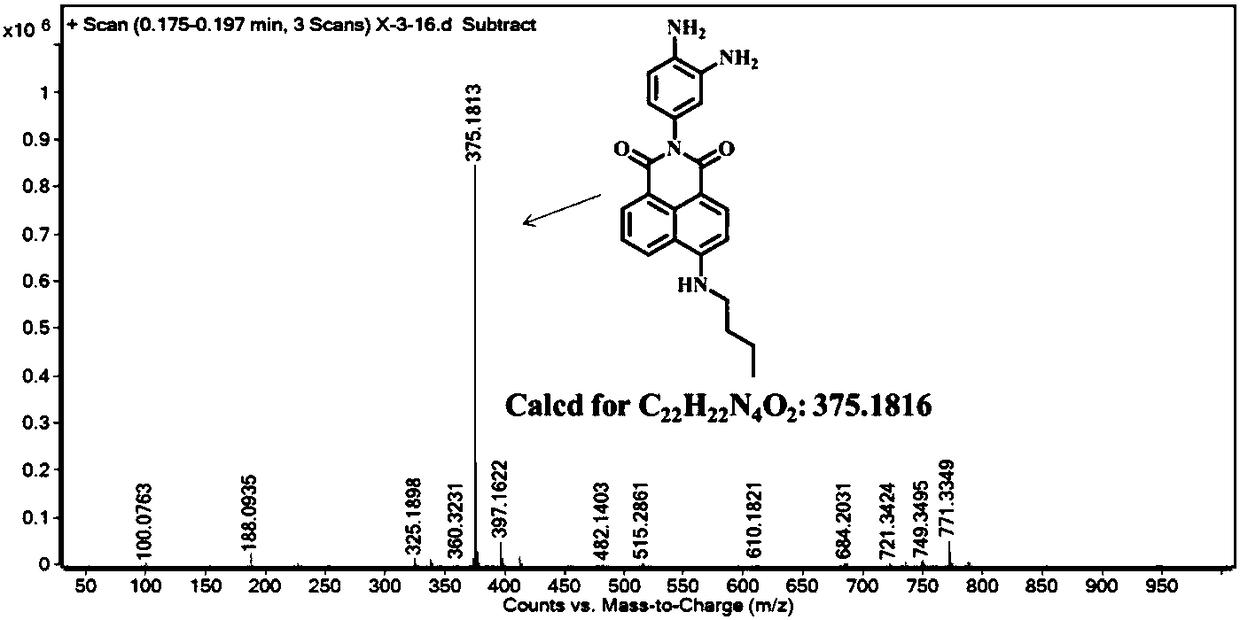
[0037] Example 1 Synthesis of Fluorescent Probes
[0038] (1) Synthesis of compound 4-bromo-N-(3-nitro-4-aminophenyl)naphthalimide (3):
[0039]
[0040] In a 100 mL round bottom flask, add 1 mmol of 4-bromo-1,8-naphthalic anhydride (1), 1 mmol of 2-nitro-1,4-phenylenediamine (2), and then add 10 mL of ethanol , heated to reflux for 5-6 h, cooled to room temperature, filtered under reduced pressure, the filter cake was washed 2-3 times with ethanol, and dried in vacuo to obtain light yellow solid 4-bromo-N-(3-nitro-4-aminobenzene base) naphthalimide (3). Yield: 91%. The product is directly carried out to the next step without purification;
[0041] (2) Synthesis of compound 4-n-butylamine-N-(3-nitro-4-aminophenyl)naphthalimide (5):
[0042]
[0043] Mix 0.2 mmol of compound 4-bromo-N-(3-nitro-4-aminophenyl)naphthalimide (3) with 1 mmol of n-butylamine (4), and heat in 20 mL of ethylene glycol monomethyl ether Reflux overnight, then cool to room temperature, filter u...
Embodiment 2
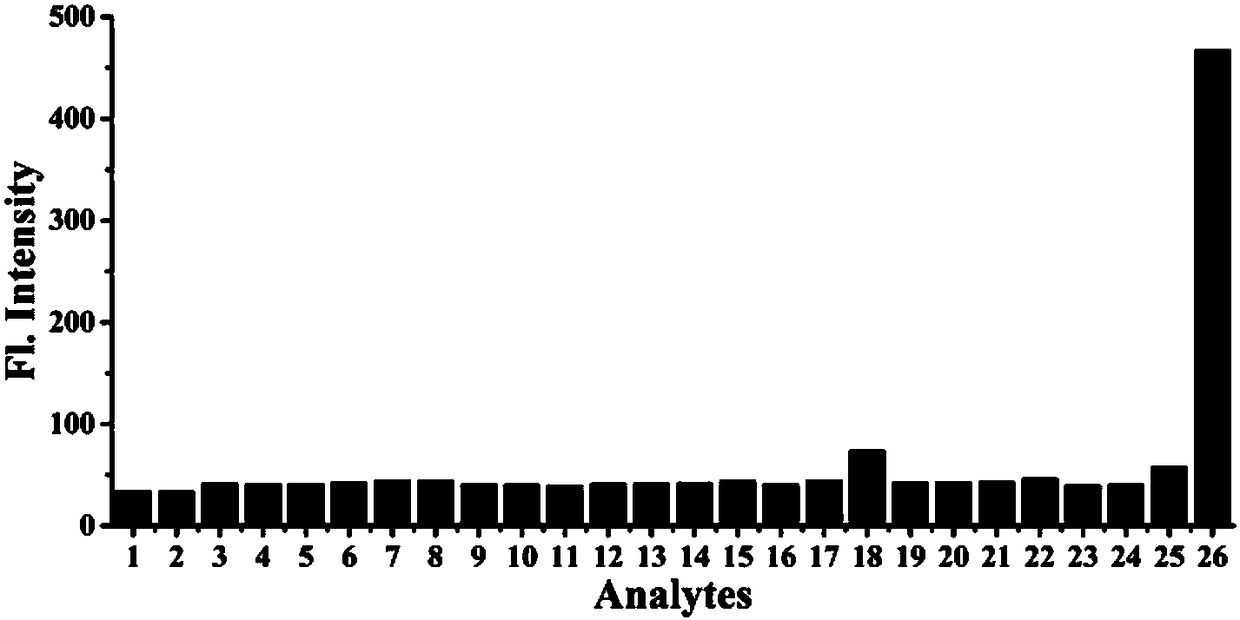
[0051] Example 2 Selectivity of Fluorescent Probes to Different Molecules or Ions
[0052] Prepare 5 mL of calcium chloride, magnesium chloride, sodium nitrate, sodium nitrite, sodium bisulfite, sodium sulfide, sodium sulfate, ferrous sulfate, potassium iodide, sodium bromide, sodium hypochlorite, hydrogen peroxide, tert-butyl Hydrogen peroxide, cysteine, homocysteine, glutathione, nitric oxide, glucose, 2,4-dinitrobenzene, sodium pyruvate, formaldehyde, acetaldehyde, chloral, The PBS mother solution of glyoxal and methylglyoxal and the fluorescent probe NB-MGO mother solution synthesized in Example 1 at a concentration of 1 mM were used for later use.
[0053] Add 25 μL probe mother solution, 225 μL DMSO and each ion (or amino acid) solution, and use phosphate buffer PBS to make the volume to 5 mL, the concentration of the test ion (or amino acid) is 2.5 mM, and the concentration of reactive oxygen and nitrogen is 100 mM, the concentration of aldehydes and ketones is 100 μM....
Embodiment 3
[0054] Example 3 Fluorescence titration detection of probe NB-MGO by different concentrations of methylglyoxal
[0055] Prepare 10 mL of an aqueous solution with a concentration of 100 mM methylglyoxal and a mother solution of the fluorescent probe NB-MGO synthesized in Example 1 with a concentration of 1 mM as backup.
[0056] Prepare the probe at a concentration of 5 μM, interact with different concentrations of methylglyoxal (0-200 μM), and perform fluorescence detection (λex = 440 nm, λem = 543 nm), such as Figure 4 Shown fluorescence titration result, along with the increase of methylglyoxal concentration, reaction system fluorescence intensity increases gradually, calculates fluorescence intensity in each system, establishes fluorescence intensity and methylglyoxal concentration standard curve ( Figure 5 ). Depend on Figure 5 It can be seen that as the concentration of methylglyoxal increases, the fluorescence intensity of the reaction system increases gradually. Wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com