Extraction method, product and application of caffeoylquinic acid compounds
A technique of caffeoylquinic acid and extraction method, applied in the field of natural medicinal chemistry, can solve the problems of frequent subcutaneous administration, hepatitis B virus infection threatening public health, drug resistance, etc., and achieves significant anti-hepatitis B virus effect and normal cytotoxicity. Low, moderately effective doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The extraction of four kinds of new natural caffeoylquinic acid compounds of embodiment 1
[0083] At room temperature, take 6.5kg of dried honeysuckle, use 75% ethanol to carry out multiple cold soaking and percolation extraction, combine the extracts, then recover the solvent under reduced pressure at 55°C, and concentrate to obtain 1500g of ethanol extract; The ethanol extract was suspended in water, and then extracted with cyclohexane, ethyl acetate, and n-butanol respectively to obtain 130.3g cyclohexane extract LJH, 93.7g ethyl acetate extract LJE, and 199.8g n-butyl Alcoholic extract LJB.
[0084] Get 83.0g of said ethyl acetate extract LJE, dissolve it with chloroform and methanol, mix the sample with 120g column chromatography silica gel (100-200 mesh), dissolve the silica gel column with dichloromethane, and equilibrate with dichloromethane The column does not drop down to the silica gel surface, and the sample is loaded by dry method, and then successively u...
Embodiment 2
[0090] The characterization of embodiment 2 caffeoylquinic acid compounds a, b, c, d
[0091] Characterization of caffeoylquinic acid compound a
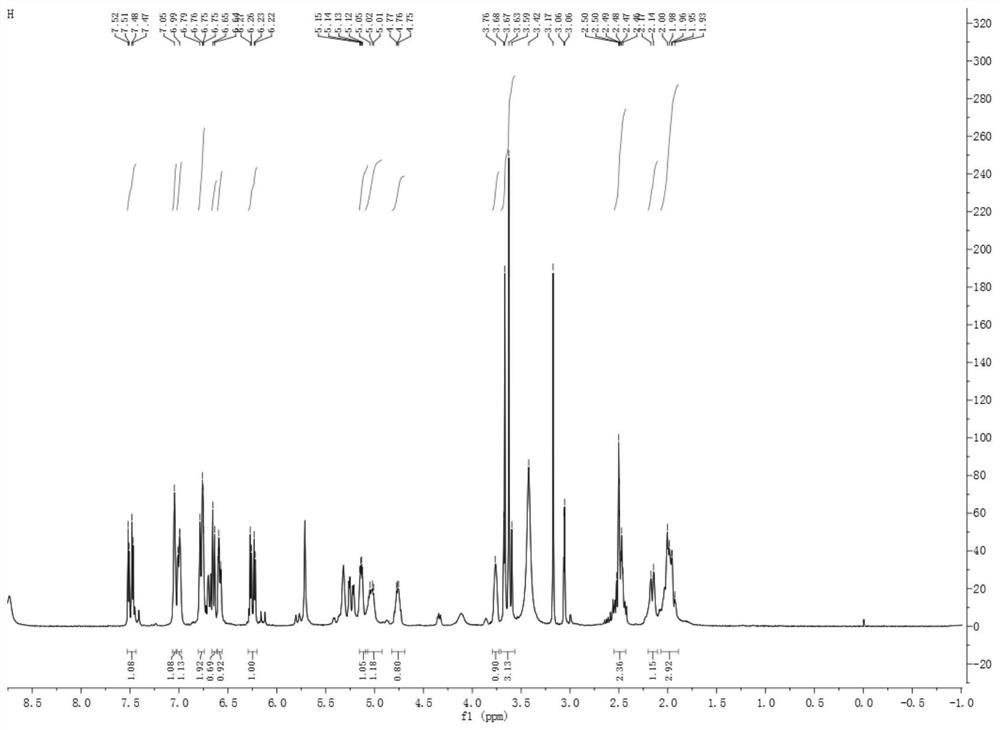
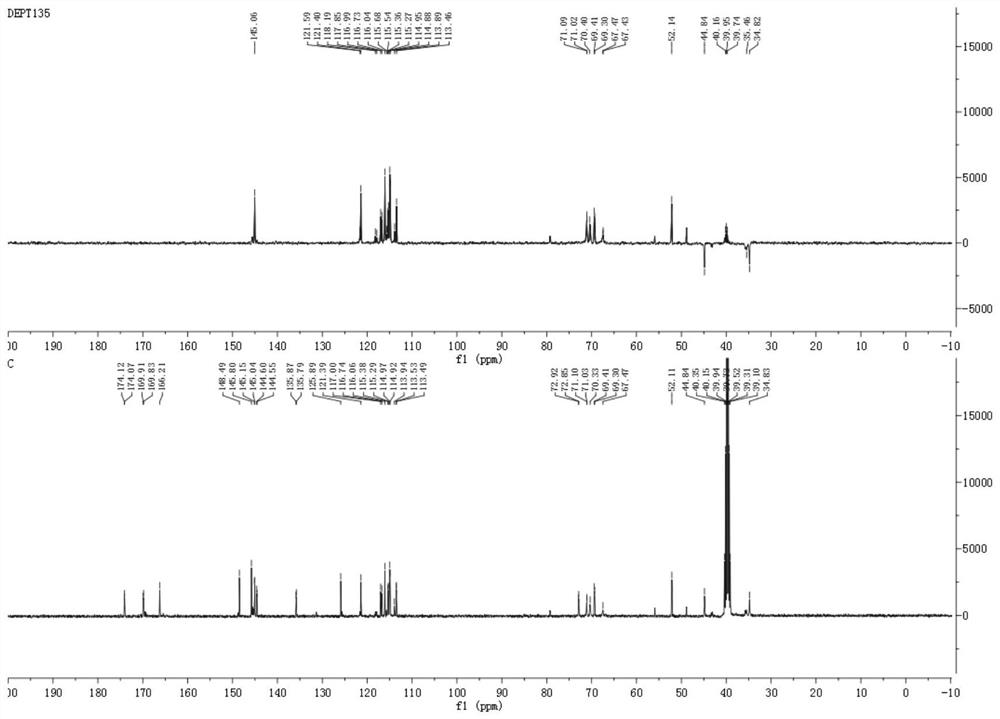
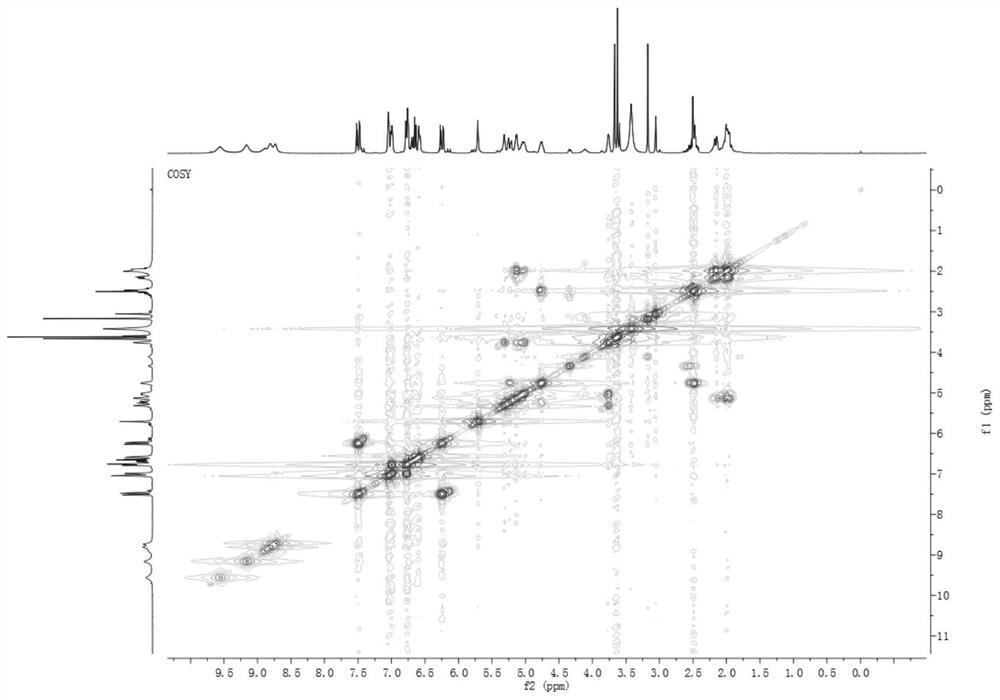
[0092] The caffeoylquinic acid compound a provided by the present invention is a yellow amorphous powder, see Figure 7 , HR-ESI-MS (negative) gives quasi-molecular ion peak [M-H] - m / z 547.1627, suggesting a molecular weight of 548; binding 1 H and 13 C NMR confirmed its molecular formula as C 26 h 28 o 13 , with a calculated unsaturation of 18.
[0093] see Figure 1~6 , combined with Table 1, in 1 H NMR, δ H 3.76(1H,s), 5.03(1H,m), 5.14(1H,m) and δ H 1.98(3H,m) and 2.16(1H,d,J=11.0Hz) and δ H 3.60 (3H, m) suggests that the compound is a 4,5-disubstituted methyl quinic acid derivative, 13 δ on C NMR C 72.90(C-1), 35.46(C-2), 67.47(C-3), 71.07(C-4), 70.33(C-5), 34.82(C-6), 174.10(C-7), 52.11 (7-OCH 3 ) displacement signal also confirmed the above judgment. also, 1 δ of ABX system on H NMR H 7.05(1H, s), 7.00...
Embodiment 3
[0107] Example 3 In vitro cytotoxicity test of caffeoylquinic acid compounds a, b, c, d
[0108] The inventors conducted an in vitro cytotoxicity test on four natural caffeoylquinic acid compounds, and the cell lines used were HepG 2 cells and HepG 2.2.15 cells. HepG 2.2.15 cells are HepG 2 cells stably transfected with HBV genes, carrying the whole HBV genome, so they can carry out virus replication and stably secrete infectious virus particles, HBsAg and HBeAg.
[0109] MTT method: Inoculate HepG 2 cells and HepG 2.2.15 cells in a 96-well cell culture plate, 200 μL per well (containing 10×10 4 tumor cells), at 37°C, 5% CO 2 In the incubator, and in the DMEM medium containing 10%FBS, culture 24h, add the compound of the present invention (being caffeoylquinic acid compound a , b, c, d), continue to cultivate for 48h; add 20μL of MTT (5mg / mL) 4h before the end of the experiment, and continue to culture at 37°C, 5% CO 2 Incubate under the conditions for 4 hours, add 150 μL o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com