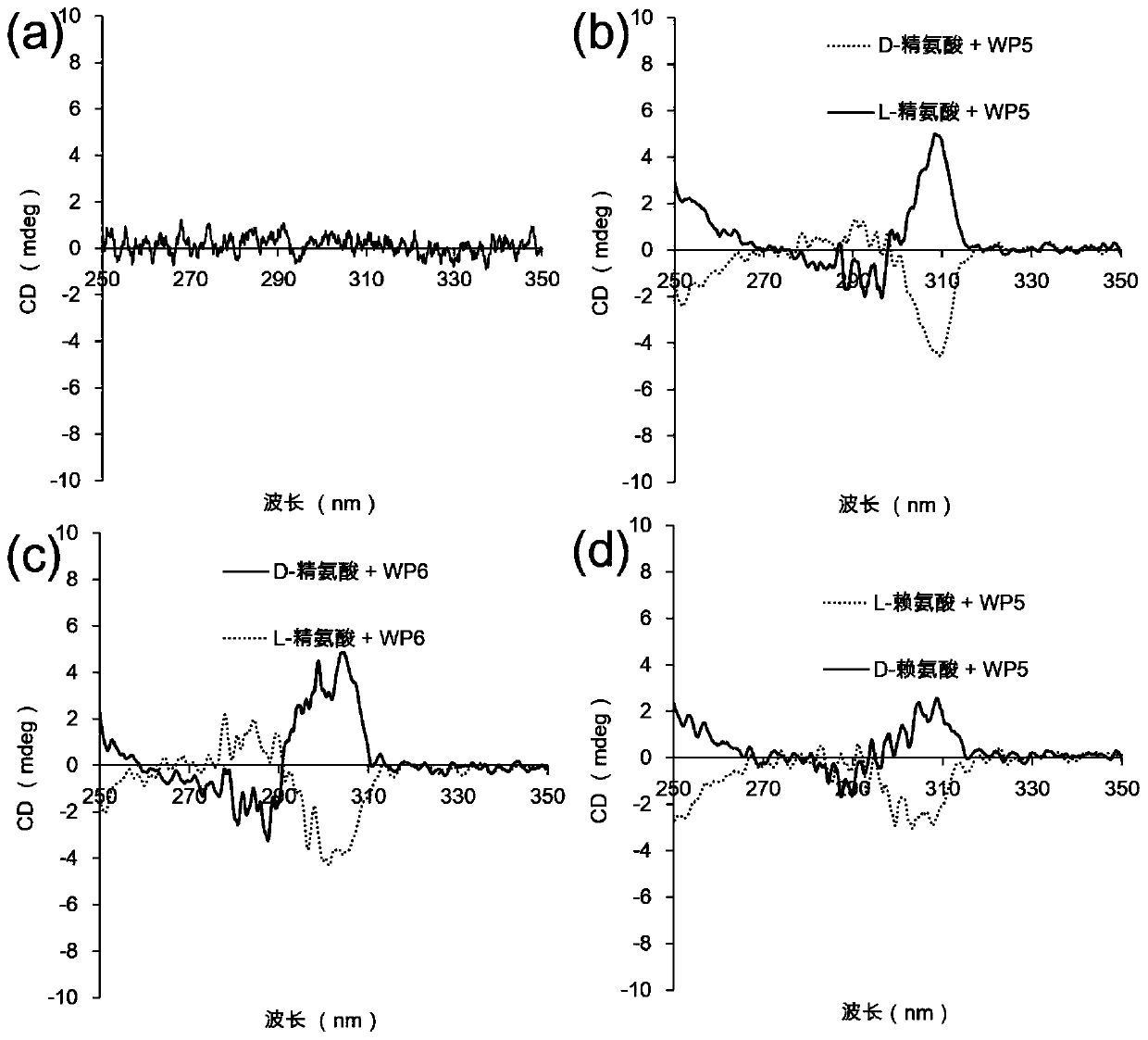
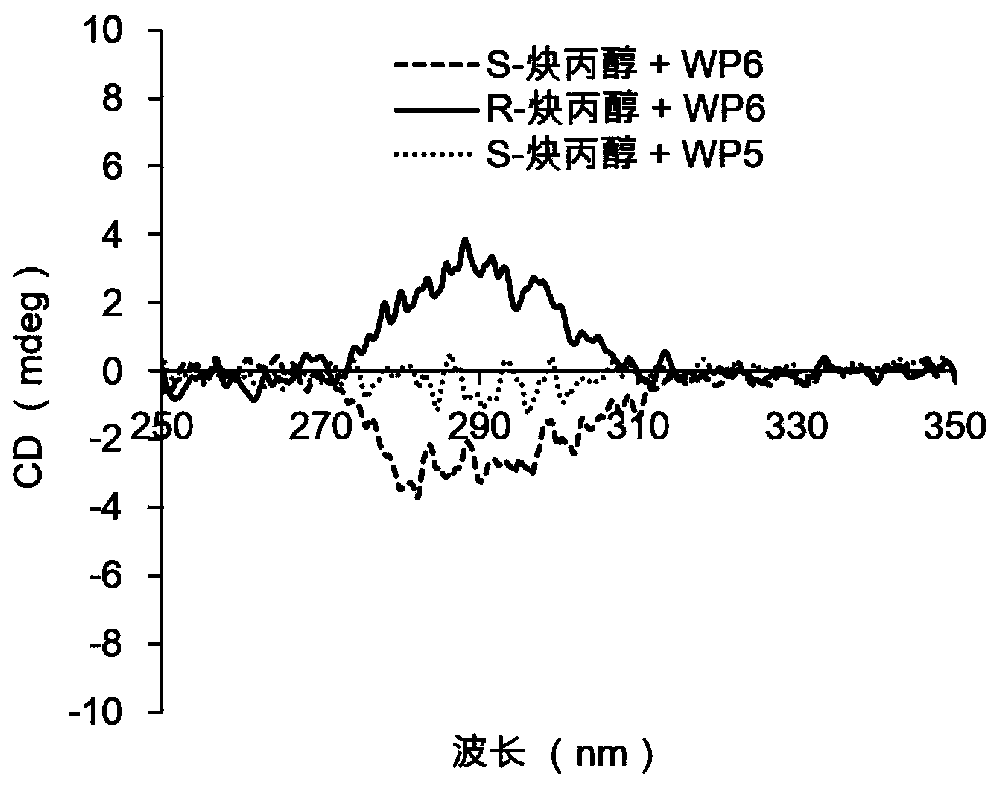
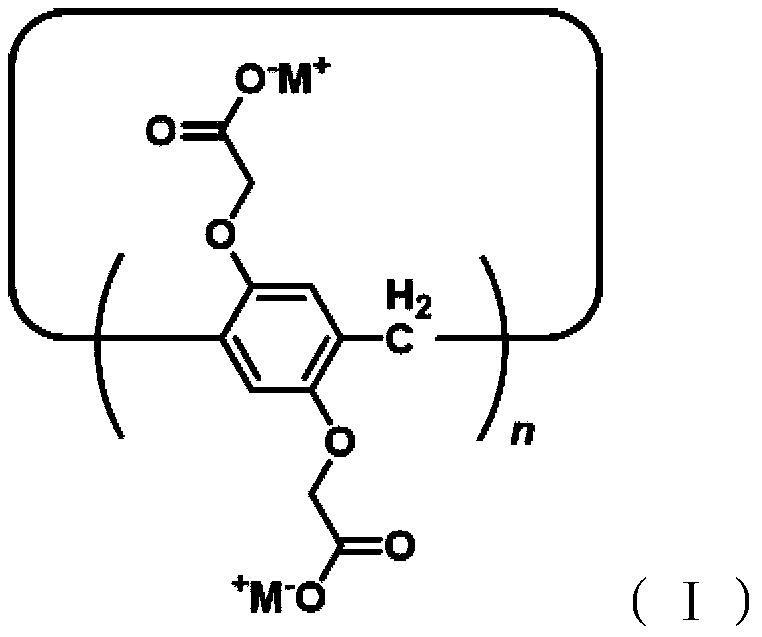
Application of a water-soluble pillar aromatic hydrocarbon chiral amplifier
A technology of water-soluble pillar aromatic hydrocarbons and amplifiers, which is applied in the preparation of organic compounds, material analysis through optical means, instruments, etc., can solve the problems of increasing test costs and long duration, and achieves simple operation, low synthesis cost, and with convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of percarboxylammonium salt pillar[5]arene WP5:
[0043]
[0044] (1) Weigh 2.40g of perhydroxy column [5]arene P5-1 in a 250mL round bottom flask, add 10mL of ethyl chloroacetate, 30g of anhydrous potassium carbonate, 100mL of acetonitrile, under the protection of argon atmosphere, at 85 The reaction was stirred at ℃ for 48h. After the reaction, filter, spin dry the solvent, dissolve the resulting solid in 200 mL of dichloromethane, wash with 300 mL of distilled water, dry over anhydrous sodium sulfate, filter, and separate by silica gel column chromatography, eluents are petroleum ether and acetic acid The mixed liquid with ethyl ester volume ratio of 3:1 was concentrated to obtain 0.62 g of full ester group column[5]arene P5-2.
[0045](2) Weigh 0.15 g of the full ester group [5] arene obtained in step (1) into a 100 mL round bottom flask, add 0.30 g of NaOH solid, 20 mL of ethanol, and 20 mL of distilled water in sequence, and react at 90 to 95 ° C for...
Embodiment 2
[0051] Synthesis of full carboxyl sodium salt column [5] arene WP5-1:
[0052]
[0053] (1) Weigh 0.56 g of percarboxylate sodium salt column [5]arene WP5-OH obtained in step (2) of Example 1 into a 50 mL round bottom flask, add 0.3 g of NaOH solid, 30 mL of distilled water, and stir at room temperature for 13 h. The pH was adjusted to about 6.5 with hydrochloric acid solution, and the distilled water in the system was spin-dried under reduced pressure to obtain 0.62 g of percarboxylate sodium salt column[5]arene WP5-1.
[0054] The characterization data of the product full carboxyl sodium salt column [5] aromatic hydrocarbon WP5-1 prepared in this embodiment is as follows:
[0055] 1 H NMR (400MHz,D 2 O,298K,ppm):δ6.61(s,10H),4.21(s,20H), 3.72(br,10H); 13 CNMR (100MHz,D 2 O, 298K, ppm): δ177.8, 151.2, 130.9, 117.7, 69.1, 30.9.
Embodiment 3
[0057] Synthesis of percarboxyammonium salt pillar[6]arene WP6:
[0058]
[0059] (1) Weigh 1.50g of perhydroxy column [6]arene P6-1 in a 250mL round bottom flask, add 10mL of ethyl chloroacetate, 30g of anhydrous potassium carbonate, 100mL of acetonitrile, under the protection of argon atmosphere, in The reaction was stirred at 90°C for 50h. After the reaction, filter and spin dry the solvent, dissolve the resulting solid in 200mL of dichloromethane, dry with 300mL of distilled water and anhydrous sodium sulfate, filter, and separate by silica gel column chromatography, the eluent is petroleum ether and ethyl acetate The mixed solution with a volume ratio of 3:1 was concentrated to obtain 0.33 g of full-ester-based column[6]arene P6-2.
[0060] (2) Weigh 0.16g of the full-ester-based columnar[6]arene P6-2 obtained in step (1) into a 100mL round bottom flask, add 0.35g of NaOH solid, 20mL of ethanol, and 20mL of distilled water in sequence, and react at 95°C for 13h , coo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com