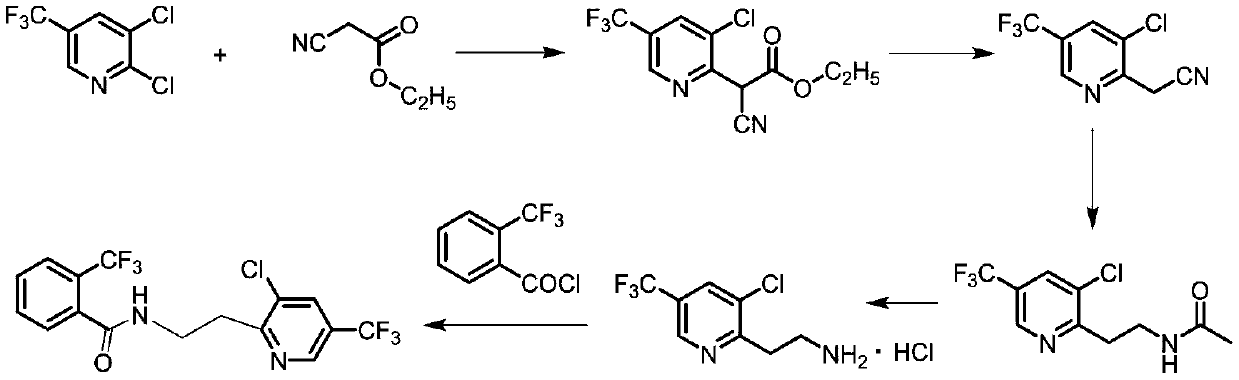
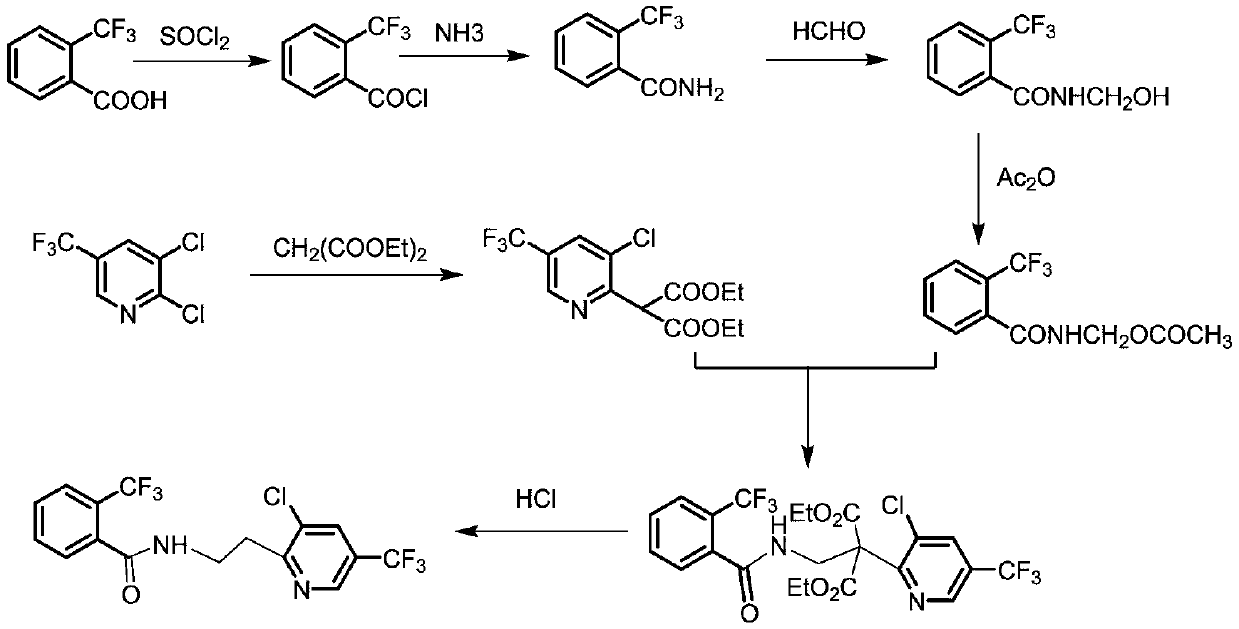
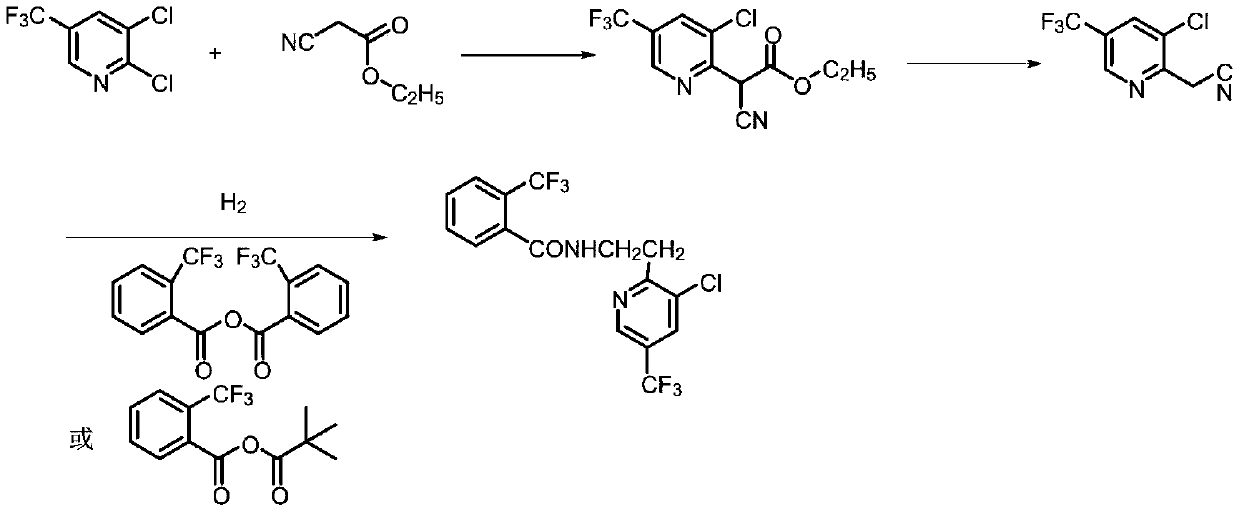
Synthesis method of fluopyram
A technology of fluopyram and fluopyram, which is applied in the field of fluopyram synthesis, can solve the problems of many side reactions in the synthesis of intermediate products, high decarboxylation temperature, and long reaction time, and achieve good industrial value and convenience The effect of recycling and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Put DCTF54.3g (0.25mol, 99.5%, 1.0eq), NMP200mL, potassium hydroxide solid 24.8g (0.375mol, 85%, 1.5eq) into a 500mL four-necked flask, and heat up to 70-80°C. Add 34.3g of ethyl cyanoacetate (0.3mol, 99% industrial product, 1.2eq) dropwise to the system, there is exothermic phenomenon, the temperature is controlled at 70-80°C, the dropwise addition is completed in about 0.5h, and the reaction is kept at 70-80°C 2h, HPLC monitors that the reaction of raw materials is complete.
[0030] Add 76.0g of concentrated hydrochloric acid (0.75mol, 36%, 3.0eq) dropwise to the system, there is a slight temperature rise phenomenon, the system emits a large amount of white smoke, a yellow solid is generated, and tail gas is released, and the system is continued to heat up to reflux reaction for 5h, HPLC detects that the reaction of the intermediate is complete, and the system is a brown-yellow suspension.
[0031] After the system was cooled down, the solvent was desolvated under r...
Embodiment 2
[0033] Into a 500mL autoclave, 34.3g (0.15mol, 96.6%, 1.0eq) of 2-acetonitrile-3-chloro-5-trifluoromethylpyridine and 50.9g (0.18mol, 97%, 1.2eq) of mixed acid anhydride were added, 5% Pd / C dry basis 1.7g, toluene 100mL, close the autoclave, nitrogen replacement three times. Pass hydrogen to the system to 1.5MPa, heat up to 20-30°C and keep it warm for reaction, keep the pressure at 1.5-2.0Mpa for 10h, and stop the ventilation if the pressure does not drop.
[0034] After pressure relief and exhaust, sample HPLC to monitor the conversion of raw materials, filter the reaction solution, rinse with an appropriate amount of toluene, and combine the mother liquor and washing solution. Add dropwise 5% sodium carbonate aqueous solution to the mother liquor to neutralize to pH = 8-9, separate layers, and desolventize the organic layer under reduced pressure to obtain 55.9 g of the crude product of fluopyram, the target product, with a content of 87.8% and a yield of 82.5%. The crude ...
Embodiment 3
[0037] Into a 500mL autoclave, 34.3g (0.15mol, 96.6%, 1.0eq) of 2-acetonitrile-3-chloro-5-trifluoromethylpyridine and 44.5g (0.158mol, 97%, 1.05eq) of mixed acid anhydride were added, Raney nickel 3.4g, chlorobenzene 100mL, close the autoclave, nitrogen replacement three times. Pass hydrogen to the system to 1.5MPa, heat up to 50-60°C and keep it warm for reaction, keep the pressure at 1.5-2.0Mpa for 12 hours, stop the ventilation if the pressure does not drop.
[0038] After pressure relief and exhaust, sample HPLC to monitor the conversion of raw materials, filter the reaction solution, rinse with an appropriate amount of toluene, and combine the mother liquor and washing solution. 5% sodium hydroxide aqueous solution was added dropwise to the mother liquor to neutralize to pH = 8-9, the layers were separated, and the organic layer was precipitated under reduced pressure to obtain 54.8 g of the target product fluopyram with a content of 92.6% and a yield of 85.3%.
[0039]T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com