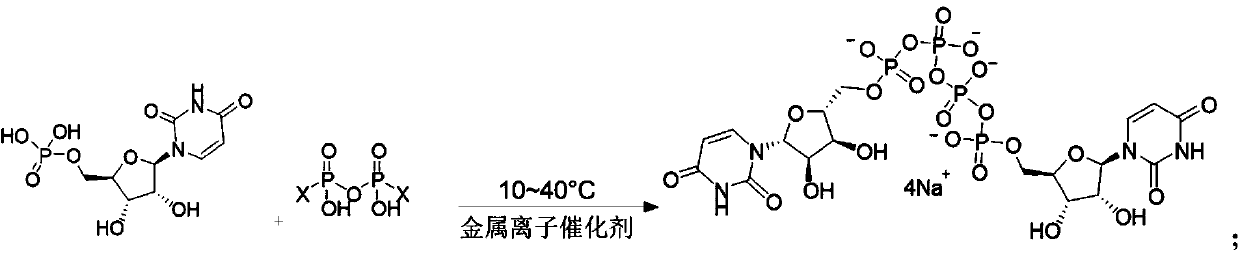
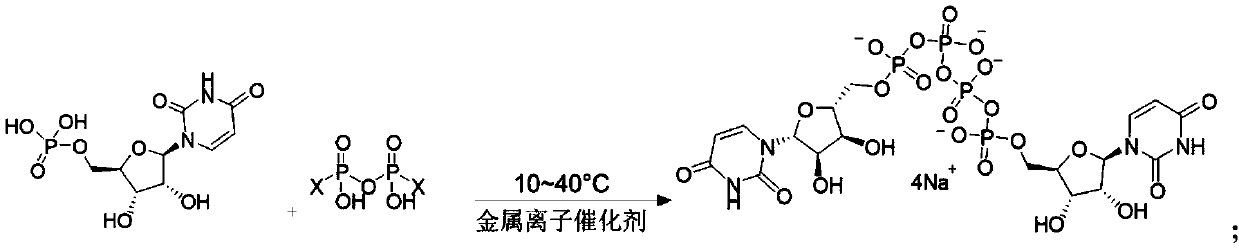
Preparation method of phosphate ester
A technology of phosphate ester and uridine monophosphate, which is applied in the field of medicine and chemical industry, can solve the problems of large metal ion catalyst equivalent, poor feasibility of industrial production, and inability to be suitable for industrial production, etc., and achieves cheap raw materials, less metal ion catalysts, and easy industrialization The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] 2 Preparation of pyrophosphate active compound
[0037] Preparation of 2-1 imidazole pyrophosphate
[0038] To a solution of triethylamine pyrophosphate (38.0 g, 0.1 mol) in DMF (380 mL), under nitrogen protection, 1,1-carbonyldiimidazole (48.6 g, 0.3 mol) was added as an activator, stirred at room temperature for 2 h, and water ( 5.4 g, 0.3 mol) was added to the reaction liquid, stirred at room temperature for 10 min, then vacuum pumped under reduced pressure, and stirred for 10 min to obtain a DMF solution of imidazole pyrophosphate (II).
[0039] Preparation of 2-methylimidazole 2-2-pyrophosphate
[0040] To a solution of triethylamine pyrophosphate (19.0g, 0.05mol) in DMF (190mL), under nitrogen protection, add 1,1-carbonylbis(2-methylimidazole) (57.1g, 0.15mol) as an activator, at room temperature After stirring for 2 hours, water (2.7 g, 0.15 mol) was added to the reaction liquid, stirred at room temperature for 10 minutes, then vacuum pumped under reduced press...
Embodiment 1
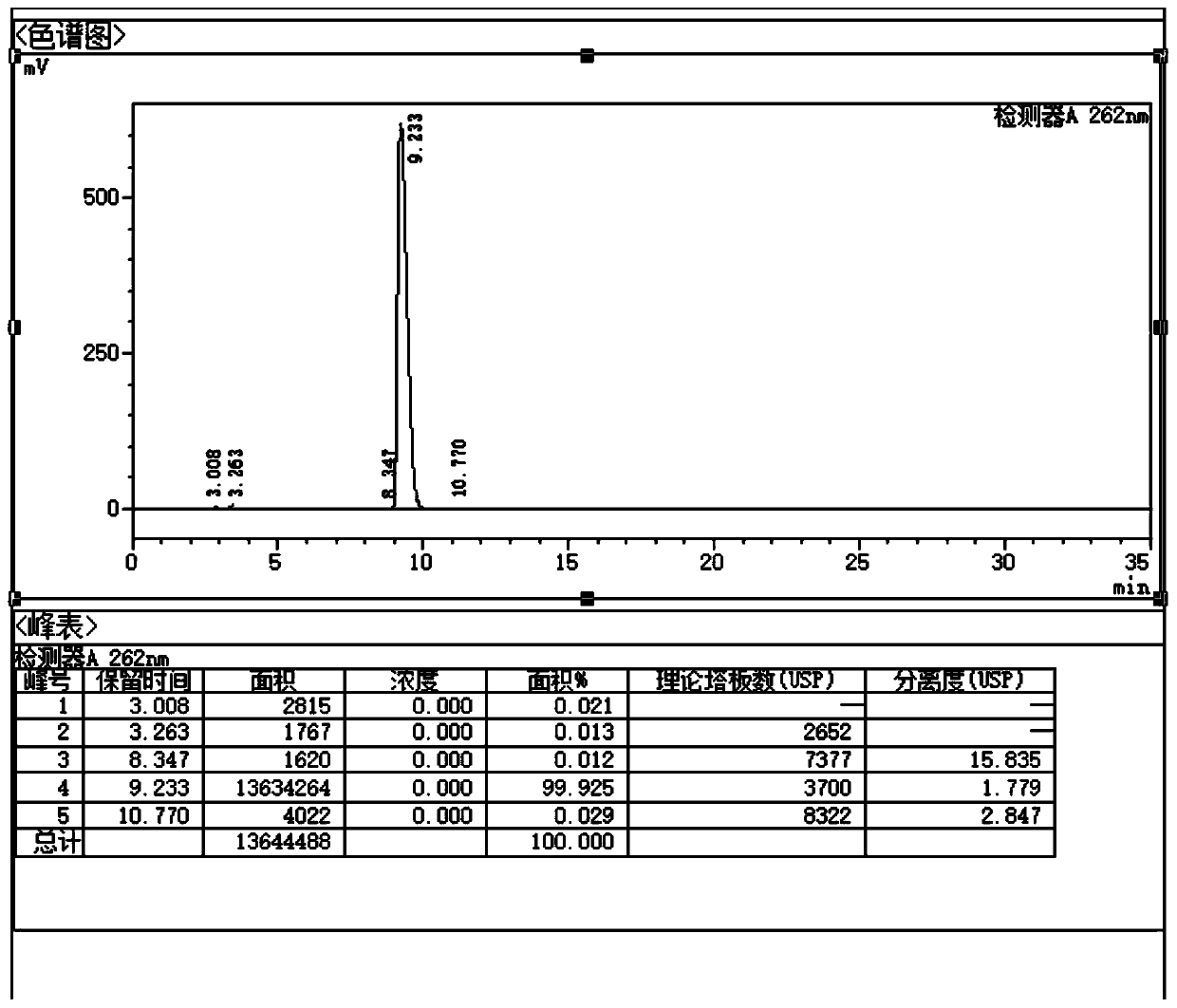
[0047] To the DMF solution (380 mL) of imidazole pyrophosphate (III, 0.2 mol) prepared in 2-1, add the DMF solution (390 mL) of uridine monophosphate (UMP, 0.4 mol) and calcium chloride (11.0 g, 0.1mol), stirred and reacted at 30°C for 4h, and the reaction liquid was taken and sent to HPLC for detection.
[0048] Add ethyl acetate (700mL) and water (600mL) to the reaction solution and stir for 10 minutes, separate and retain the water phase, add saturated aqueous sodium carbonate solution to the water phase to adjust the pH to about 10, filter, discard the filter cake, add ethanol to the filtrate (1200mL), stirred for 12h, filtered and discarded the mother liquor.
[0049] The filter cake was dissolved by adding water (400mL), and the aqueous solution was passed through an anion exchange column (Amberlite IRA-67, chlorine type), eluted with deionized water and 0.18N hydrochloric acid to remove by-products, and then washed with 0.5N sodium chloride and 0.005N hydrochloric acid ...
Embodiment 2
[0051] To the DMF solution (380mL) of imidazole pyrophosphate (Ⅲ, 0.2mol) prepared in 2-1, add the DMF solution (390mL) of uridine monophosphate (UMP, 0.4mol) and magnesium chloride (9.52g, 0.1mol) under ice-cooling ), stirred at 30°C for 4h.
[0052] Add ethyl acetate (700mL) and water (600mL) to the reaction solution and stir for 10 minutes, separate and retain the water phase, add saturated aqueous sodium carbonate solution to the water phase to adjust the pH to about 10, filter, discard the filter cake, add ethanol to the filtrate (1200mL), stirred for 12h, filtered and discarded the mother liquor.
[0053] The filter cake was dissolved by adding water (400mL), and the aqueous solution was passed through an anion exchange column (Amberlite IRA-67, chlorine type), eluted with deionized water and 0.18N hydrochloric acid to remove by-products, and then washed with 0.5N sodium chloride and 0.005N hydrochloric acid The target product was eluted with aqueous solution, and the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com