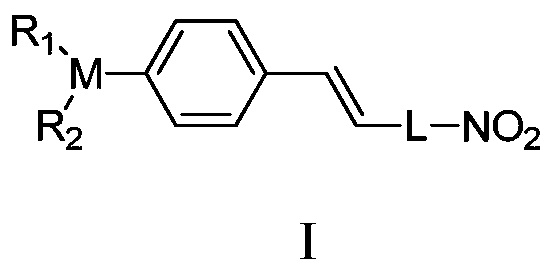
Dual-response fluorescent dye molecules
A fluorescent dye, dual-response technology, applied in the field of organic fluorescent dyes, can solve the problems of inability to achieve dual-line detection of NTR and NADH, and achieve the effects of low/non-toxic, high selective recognition, and suitable water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] On the other hand, the present invention also describes the preparation method of the above-mentioned fluorescent dye, comprising the following steps:
[0053] In the presence of a catalyst, compound a and b react according to a molar ratio of 1:1-1:10 to prepare a compound of general formula I;
[0054]
[0055] The reaction temperature is 0-200°C, the reaction time is 1-32 hours, and the reaction solvent is selected from ethanol, glacial acetic acid, ethylene glycol monomethyl ether, methanol, dichloromethane, acetone, glycerol, ethyl acetate, N-N- 2 methylformamide or its mixture; reaction catalyst is selected from piperazine, piperidine, potassium hydroxide, sodium hydroxide, pyridine, sodium methoxide, methanol-potassium hydroxide, potassium carbonate, triethylamine and 4-dimethylamino pyridine.
[0056] Wherein the molar ratio of catalyst to reactant is 1:0.05-1:0.2.
[0057] In a specific embodiment, the reaction temperature of the step is preferably 30-180°...
Embodiment 1
[0067] The synthetic route of dye compound 1-1 is as follows:
[0068]
[0069] (1) Synthesis of Dye Compound 1-1
[0070] 5mmol of a-1 (synthesis method reference: Kawaguchi, Hirobumi; Hamasaki, Ichiya; Inagaki, Yoshio. Stilbene derivative and electrophotographic photoreceptorusing it May6, 1998JP 10114728) and 6.25mmol of b-1 (synthesis method reference: Venis, Michael A. and Thomas, Emrys W. Synthesis and auxin activity of 5-substituted 1-naphthalene acetic acids. Phytochemistry, 1990, 29, 381-383) were added to a round bottom flask containing 6 mL of absolute ethanol, and then piperidine was added for catalysis. The reaction was heated to 100°C for 3 hours, then heated to 120°C for 5 hours, and then stopped. The solvent was removed by vacuum spin-drying, followed by column chromatography (petroleum ether:dichloromethane, 1:1, v / v) to obtain orange-red compound 1-1 with a yield of 50%.
[0071] (2) Characterization of Dye Compound 1-1
[0072] 1 H NMR (600MHz, CDCl3)...
Embodiment 2
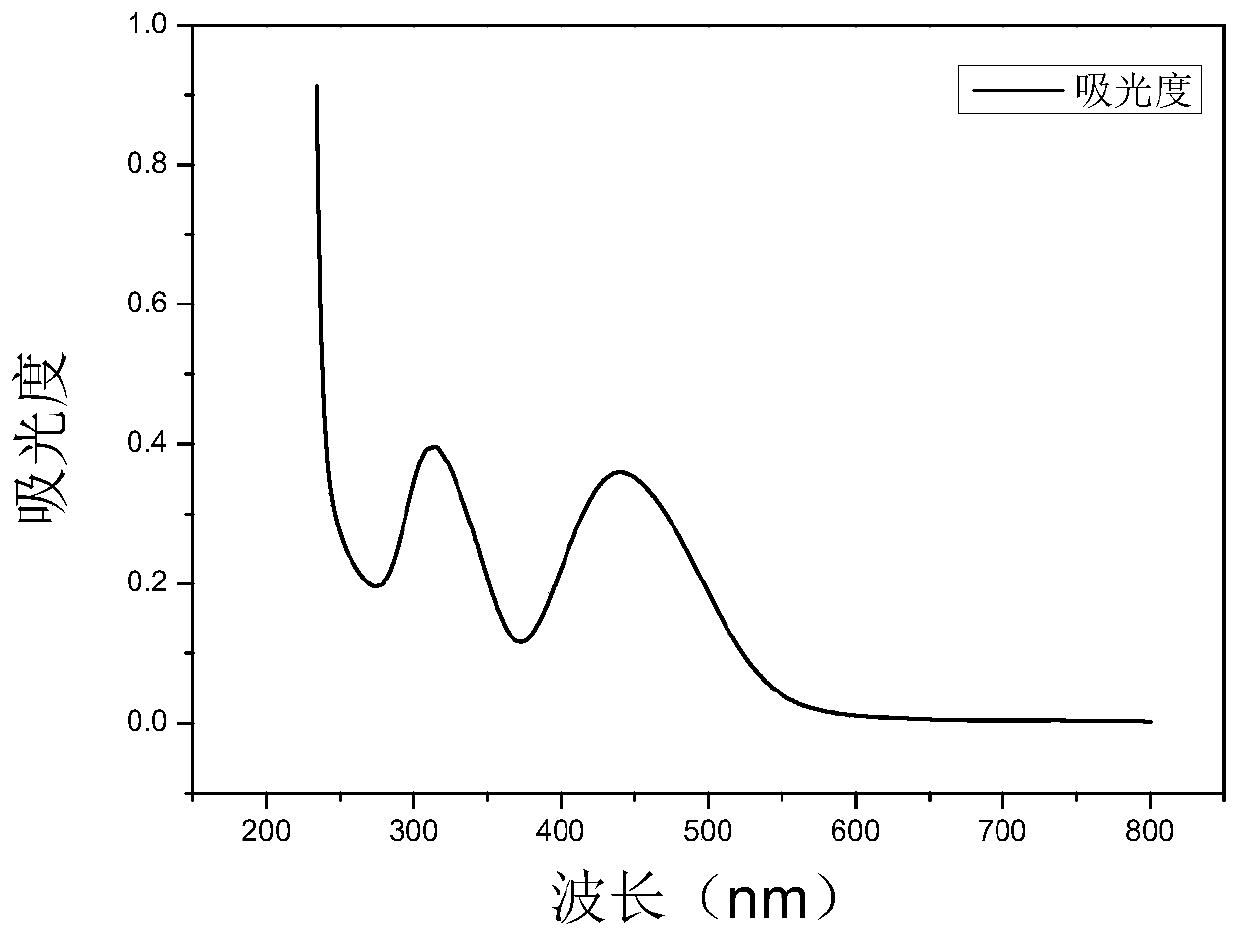
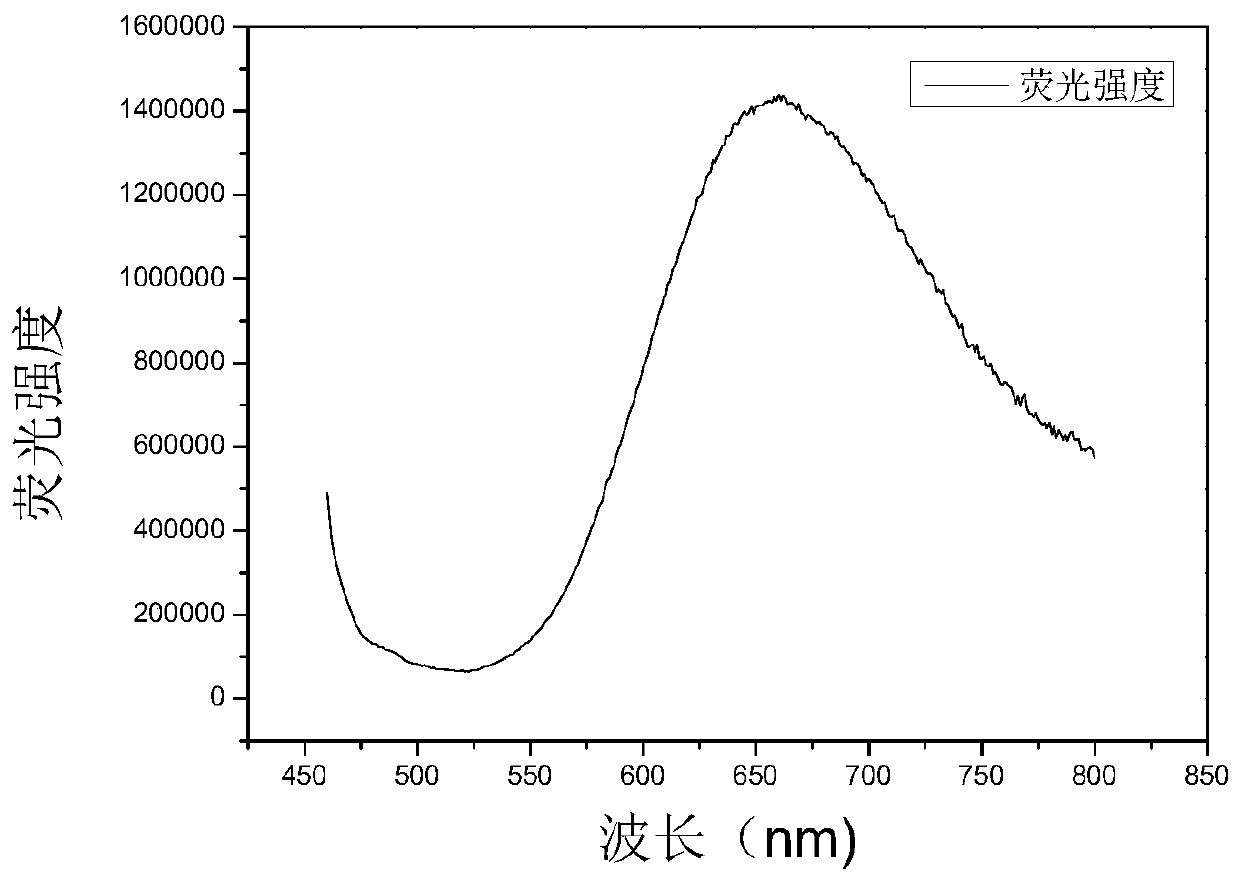
[0073] Embodiment 2: Absorption Spectrum Detection Test of Dye Compound 1-1
[0074] Take 3μL of 6mmol / L dye mother solution prepared with DMSO and add it to a four-way cuvette containing 3mL of high-purity water, measure its absorption spectrum, collect the absorbance value at a wavelength of 230-800nm, and the wavelength corresponding to the maximum absorbance value is its absorption wavelength. The result is as figure 2 shown.
[0075] The instruments used in this example are Agilent 8453 UV spectrophotometers.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com