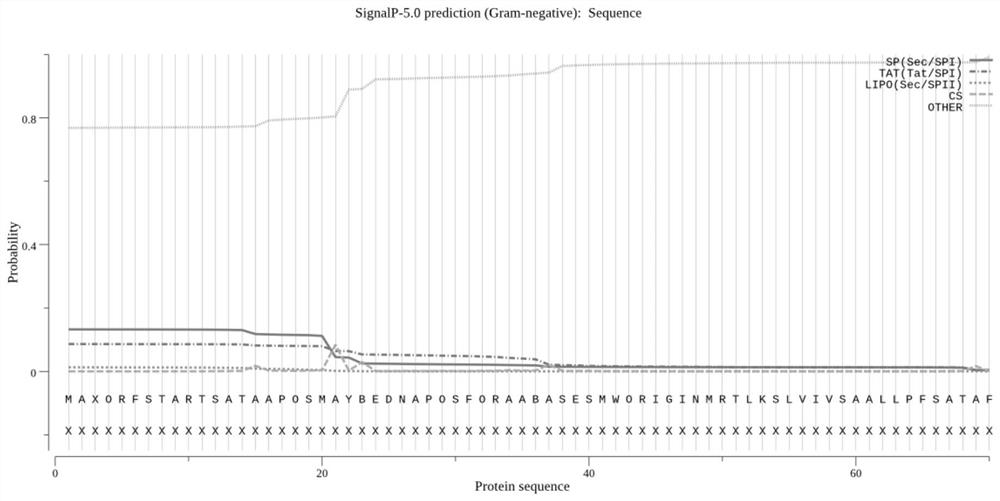
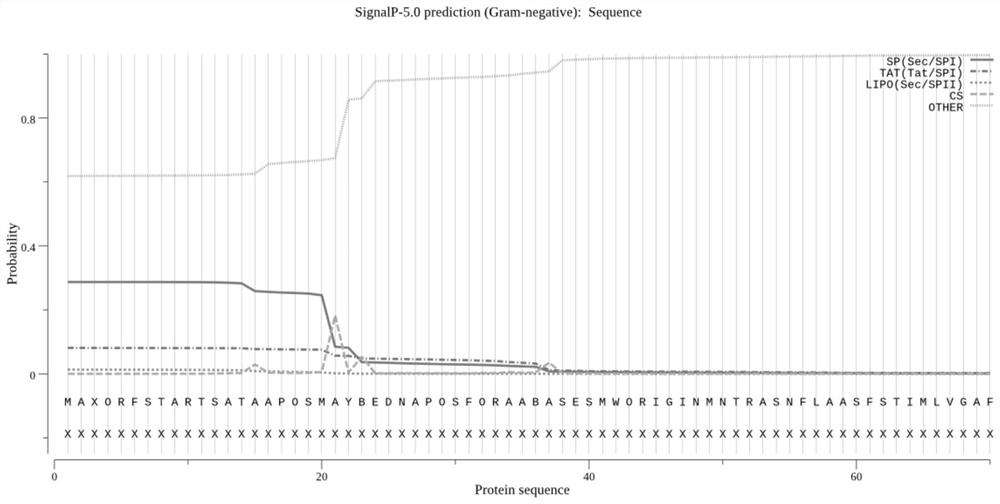
Fusion protein of brucella outer membrane protein OMP25 and periplasmic protein BP26 as well as expression and application of the fusion protein
A technology of OMP25 and Brucella, applied in the field of fusion proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Gene Design and Recombinant Plasmid Synthesis and Expression of Brucella Outer Membrane Protein OMP25 and Periplasmic Protein BP26
[0047] 1. Main reagents
[0048] DNA Quick Ligation Kit, DL 2 000 Marker, rTaq, 6×DNA loading buffer enzyme, etc. were purchased from Dalian Baoriyi Biotechnology, 30% acrylamide, ammonium persulfate (APS), SDS, TEMED, SDS-PAGE protein loading Buffer (5×), Coomassie Brilliant Blue Rapid Staining Solution, protease inhibitors, nucleic acid dyes, etc. were purchased from Shandong Sikejie Scientific Instrument Co., Ltd., plasmid extraction kits, and gel recovery kits were purchased from Beijing Biotek Biotechnology Co., Ltd. IPTG, etc. were purchased from Sigma, and Rosetta (DE3) competent cells were preserved and made by our laboratory. Other reagents were domestic or imported analytically pure.
[0049] 2. Recombinant protein gene design
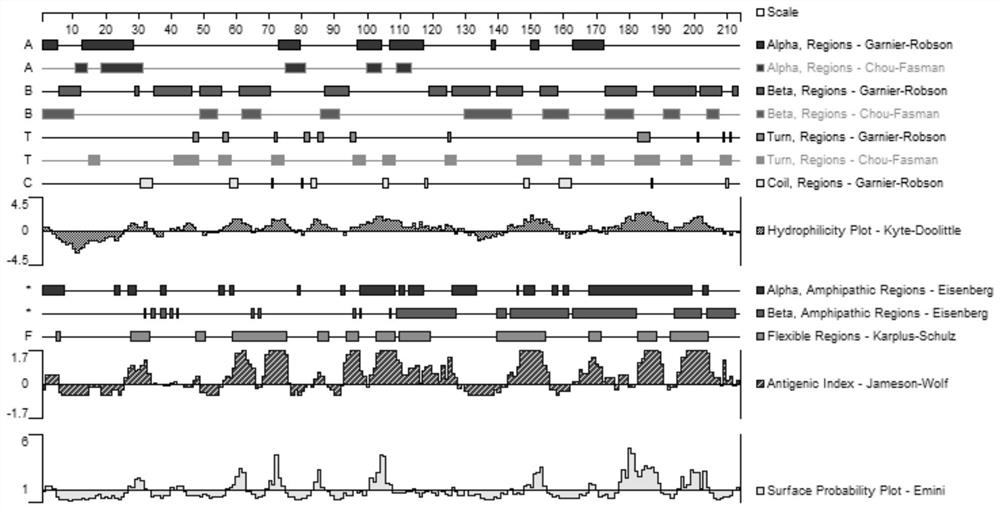
[0050]The amino acid sequences of Brucella outer membrane protein OMP25 and periplasmic protein BP2...
Embodiment 2
[0072] Fusion Protein Purification and Western Blot Analysis of Brucella Outer Membrane Protein OMP25 and Periplasmic Protein BP26
[0073] 1. Main reagents:
[0074] 30% acrylamide, ammonium persulfate (APS), SDS, TEMED, SDS-PAGE protein loading buffer (5×), Coomassie brilliant blue fast staining solution, skimmed milk powder, protease inhibitors, etc. were purchased from Shandong Sikejie Scientific Instruments Co., Ltd., His-nickel ion affinity chromatography column was purchased from GE Life Sciences, horseradish peroxidase (HRP) enzyme-labeled rabbit anti-goat * bovine secondary antibody was purchased from Henan Servi. Dialysis bags were purchased from Spectrum USA. Other reagents were domestic or imported analytically pure.
[0075] 2. Ni-column affinity chromatography purification of fusion protein
[0076] The fusion protein solution after ultrasonic cracking was purified by affinity chromatography using the AKATA protein purification system through His-nickel ion af...
Embodiment 3
[0084] Establishment and application of iELISA detection method based on fusion protein of OMP25 and BP26
[0085] 1. Main reagents
[0086] Triton-100 was purchased from Shandong Sikejie Scientific Instrument Co., Ltd., TMB was purchased from Beijing Tiangen Biochemical Technology Co., Ltd., horseradish peroxidase (HRP) enzyme-labeled rabbit anti-goat * bovine secondary antibody was purchased from Henan Servi. ELISA plates were purchased from Guangzhou Jiete Biological Filtration Products Co., Ltd. Other reagents were domestic or imported analytically pure.
[0087] 2. Establishment and optimization of fusion protein antibody iELISA detection method
[0088] 2.1 Optimum dilution of antigen and optimal dilution of serum optimization
[0089] Using the square array method, using the purified fusion protein as the coating antigen, set four dilutions of 1:25, 1:50, 1:100, 1:200, 1:400, respectively diluted with coating buffer and coated ELISA plate, 100 μL per well, choose thre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com