Detection method of glucosinolate compounds
A technology of glucosinolates and detection methods, applied in the field of analytical chemistry, which can solve the problems of small sampling volume, many detection steps of fluorescence detection method, and long separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1 sample processing
[0109] Weigh 0.20 g sample of crushed cauliflower powder (250-830 μm) after freeze-drying (freeze-drying temperature -50 ° C, time is 48 h), preheat at 75 ° C for 10 min, add 20 mL of 70 vol% methanol aqueous solution preheated to 75 ° C, Extract in a water bath at 75°C for 20 minutes. During the extraction process, oscillate and shake well every 5 minutes. After extraction, extract with ultrasound at room temperature for 10 minutes at a frequency of 40 kHz; centrifuge at 4000 rpm for 5 minutes. Take 100 μL of the supernatant in a 1.5 mL centrifuge tube, add 900 μL of 70% methanol solution, shake and shake well, filter the membrane (0.22 μm), store at 4°C until ready to use.
Embodiment 2
[0110] Embodiment 2 instrument method and standard substance preparation and preservation
[0111] 1. Instruments and equipment
[0112] Waters Acquity UPLC liquid chromatograph (Waters, USA), AB SCIEX 5500 triple quadrupole mass spectrometer (AB SCIEX, USA), vortex mixer, high-speed centrifuge, nitrogen blow-dryer, electronic analytical balance, pipette gun Wait.
[0113] 2. Materials and reagents
[0114] Standard products such as 3-butenyl glucosinolate (Gluconapin), 4-hydroxybenzyl glucosinolate (Sinalbin), and sinigrin (Sinigrin) were purchased from Shanghai Yuanye Biotechnology Co., Ltd.; chromatographically pure acetonitrile and methanol were purchased from Shanghai Titan Technology Co., Ltd.
[0115] 3. Preparation and storage of standard solution
[0116] Accurately weigh 5 mg of Gluconapin, Sinalbin, and Sinigrin standards into volumetric flasks, use ultrapure water to make up to 10 mL, and store them as standard stock solutions in the dark at -20°C. Take an app...
Embodiment 3
[0117] Embodiment 3 Qualitative analysis of glucosinolates
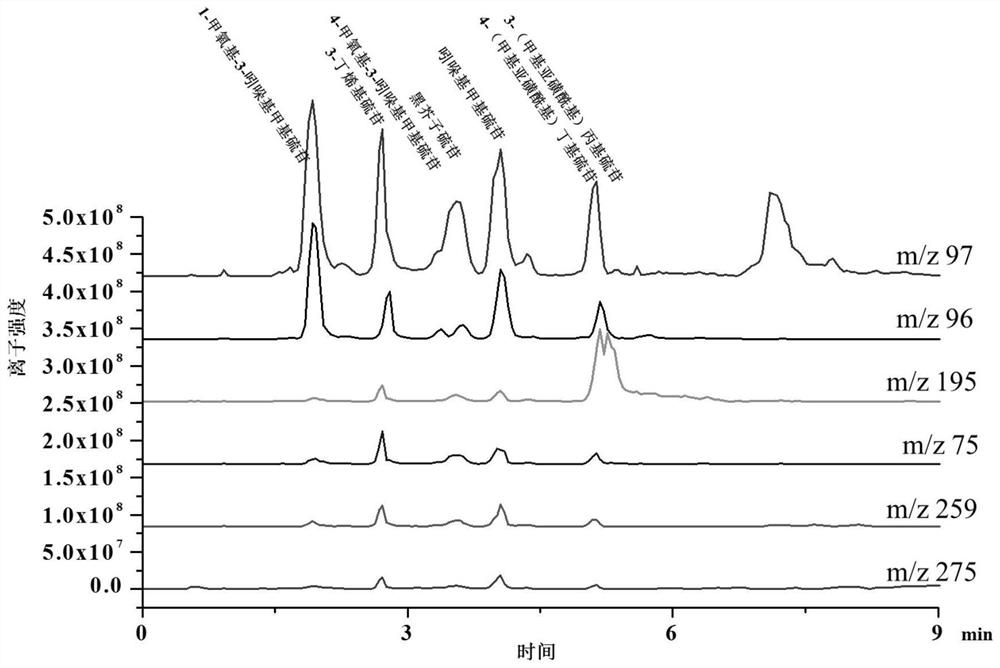
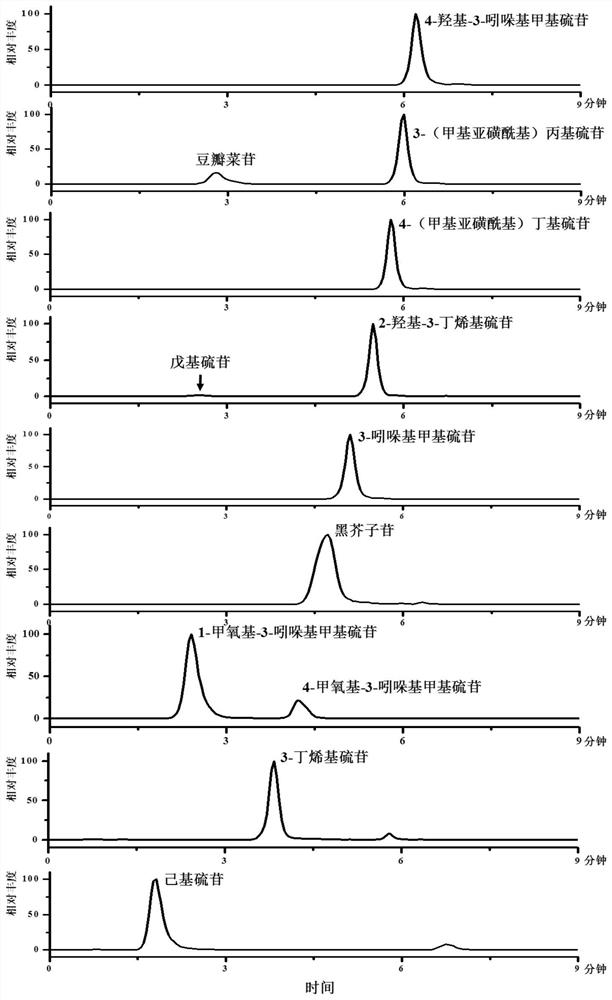
[0118] The first ultra-high liquid chromatography and mass spectrometry were used to qualitatively analyze the sample obtained in Example 1.
[0119] The parameters of the first ultra-high liquid chromatography and mass spectrometry include the first ultra-high liquid chromatography parameters and the first mass spectrometry parameters;
[0120] The first ultra-high liquid chromatography parameters include: the chromatographic column is a Merck ZIC-HILIC column, 100mm×2.1mm (i.d.), 3.5μm The temperature of the chromatographic column is preferably 40°C; the mobile phase A is acetonitrile, and the mobile phase B is 5mmol / L ammonium acetate aqueous solution; the flow rate is 0.3mL / min; the injection volume is 3μL; gradient elution conditions: 0~1min, 90% A; 1 ~ 9min, 90% ~ 65% A;
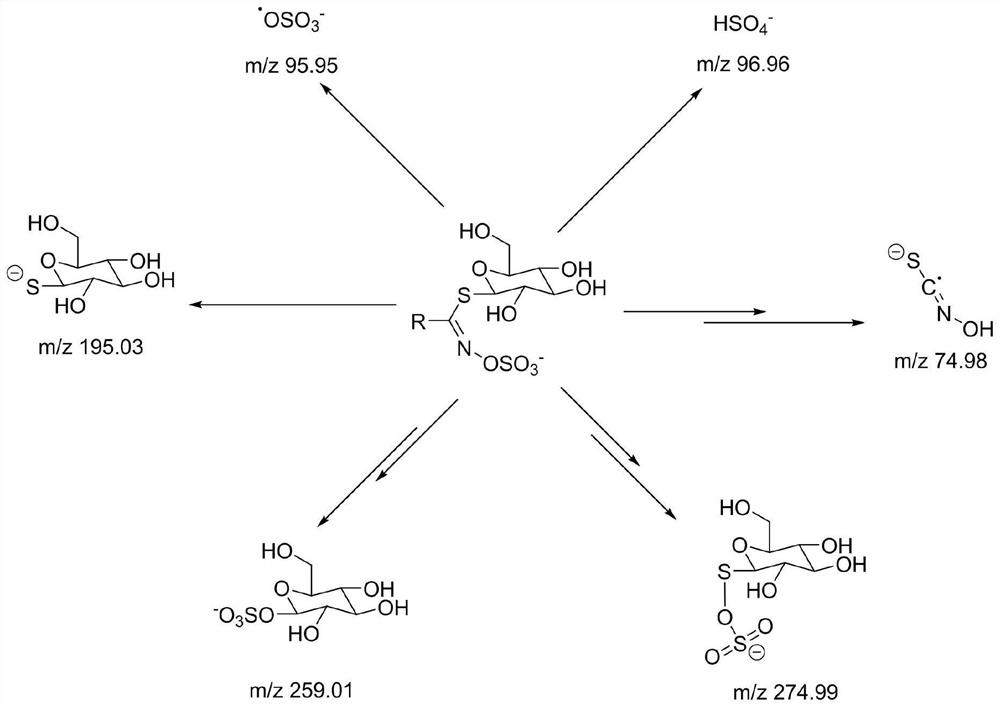
[0121] The first mass spectrometry parameters include: scan mode: precursor ion scan (precursor ion) scan mode, preferably negative ion m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com