Polyetheretherketone composite implant as well as preparation method and application thereof
A polyether ether ketone, implant technology, applied in the field of polyetherether ketone composite implants and its preparation, can solve the lack of immune activity and osteogenic activity, increase surface roughness and osteoconductivity, random experimental results Sexuality and other problems, to achieve the effect of improving immune activity and osteogenic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Preparation of PEEK and 5% DEX samples
[0080] Grind polyetheretherketone discs with a diameter of 15 mm and a thickness of 1 mm with sands of 600, 800, 1000, 1200 and 2000 in sequence, and then ultrasonically clean the polished polyether ether ketone with acetone, alcohol and deionized water in sequence clean. This pretreated sample is labeled PEEK.
[0081] A drug-loaded coating was constructed on the surface of PEEK by solvent evaporation. The selected polymer coating is polytrimethylene carbonate (PTMC), the solvent is methylene chloride, the drug is dexamethasone, and the mass ratio of the drug to the polymer is 1:5. This pretreated sample was labeled 5% DEX. The specific preparation method is as follows: first, PTMC and dexamethasone are added into the dichloromethane solution at a mass ratio of 5:1 to dissolve and mix to form a homogeneous solution, and then pour the homogeneous solution containing PTMC and dexamethasone on the surface of the PEEK s...
Embodiment 2
[0083] The preparation of embodiment 2 2KV sample
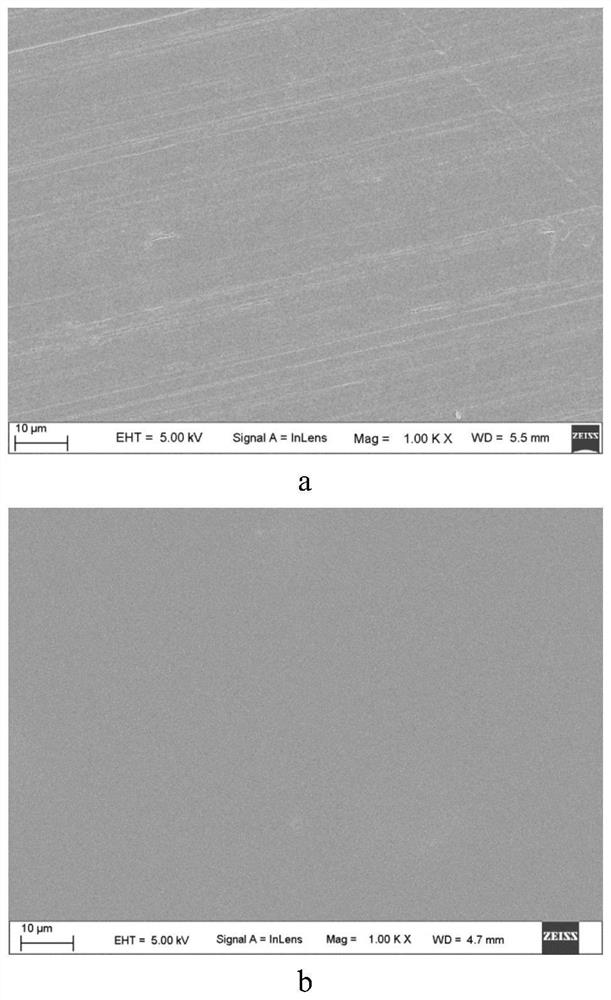
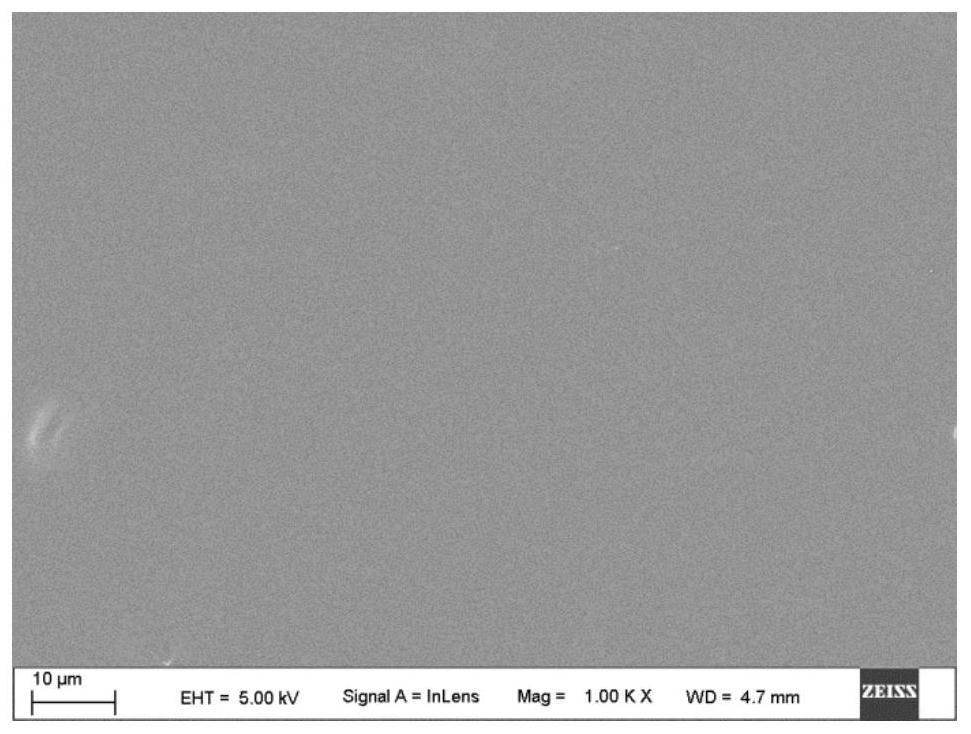
[0084] The 5% DEX in Example 1 was treated by gas plasma immersion ion implantation technology. The specific treatment process is: the background vacuum is 2×10 -3 Pa, the flow rate of the gas introduced is 60SCCM, the negative bias applied to the sample disk is 2kV, the injection pulse width is 50 microseconds, the injection pulse frequency is 50Hz, and the radio frequency power is 1000W. Wherein, the nitrogen injection time is 60 minutes, and the processed sample is called 2KV. The surface of 5% DEX after nitrogen plasma immersion ion implantation was observed by scanning electron microscope, and obtained figure 2 The photographs of the surface microstructure are shown. Depend on figure 2 It can be seen that there is no obvious difference between the surface of 5% DEX and the 2KV sample, indicating that the nitrogen plasma immersion ion implantation treatment has no obvious change on the surface morphology of 5% DEX. ...
Embodiment 3
[0085] Example 3 Preparation of 2KV-IL-10 samples
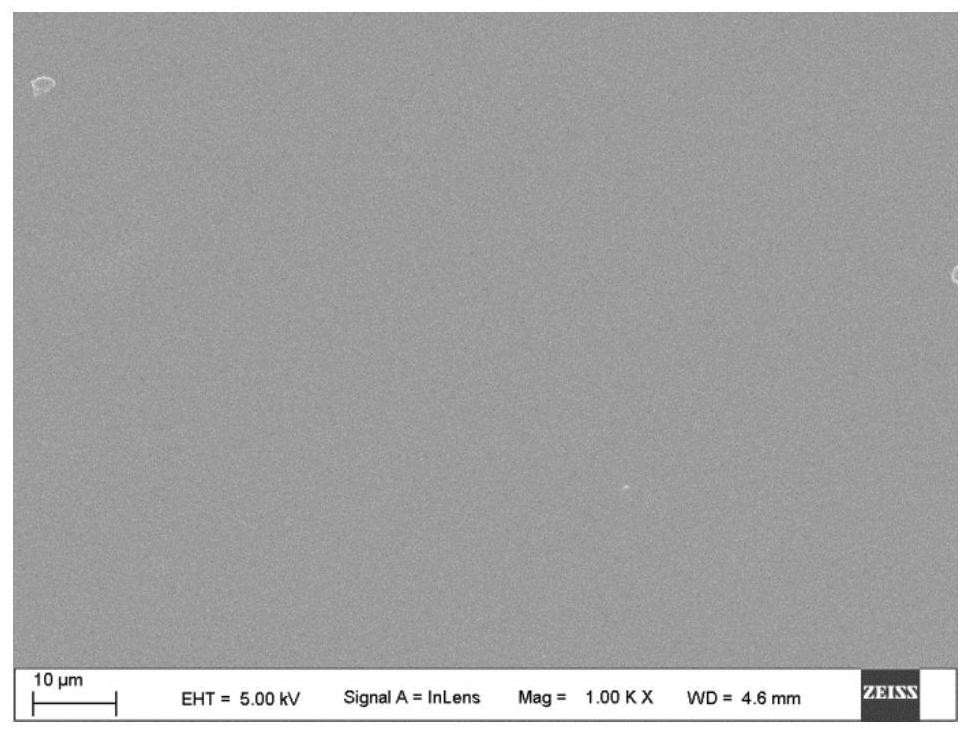
[0086] The 2KV sample modified in Example 2 was immersed in phosphate buffered saline solution (PBS) containing IL-10, wherein the concentration of IL-10 was 40ng / ml, and stored at 4°C for 24 hours. Then the sample was taken out from the IL-10 solution, and the sample was rinsed with PBS without IL-10 to remove ungrafted IL-10, and the sample was labeled as 2KV-IL-10. The microscopic morphology of the sample grafted with IL-10 was observed by scanning electron microscopy, and the results were as follows: image 3 shown. from image 3 It can be seen that the surface of the coating has no obvious change after grafting IL-10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com