Method for synthesizing phosphorus pentafluoride and preparing lithium hexafluorophosphate by solid phase method
A technology of lithium hexafluorophosphate and phosphorus pentafluoride, applied in lithium hexafluorophosphate, chemical instruments and methods, phosphorus halides/oxyhalides, etc., can solve the problems of low concentration of hexafluorophosphoric acid solution, reduce the amount of oleum, and high production costs, and achieve Good crystallization, mild reaction, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
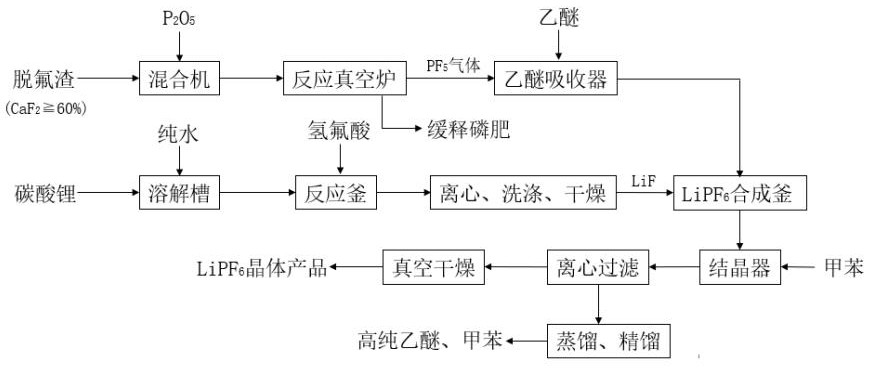
[0027] Ⅰ.PF 5 Preparation: Weigh 100g of defluorinated slag powder (CaF in defluorinated slag 2 content of 65%), 150g of phosphorus pentoxide in a sealed sample bag, after preliminary mixing, pour it into a laboratory multifunctional pulverizer and pulverize it for about 1min, put the mixture in a tube vacuum furnace, and turn on the vacuum pump to evacuate to 0.05MPa Finally, turn off the vacuum pump, turn on the heating power supply of the tube furnace, turn on the vacuum pump again after the temperature rises to 80°C, turn off the vacuum pump after the vacuum reaches 0.08MPa, and set the temperature of the tube furnace to 300°C (heating rate 5°C / min). When it reaches 300°C, react for 60 minutes, and the gas generated by the reaction is passed into a closed ether absorption bottle. The mass of ether is 60g, and PF dissolved in 5 99.2g of ether solution;
[0028] Ⅱ. Preparation of LiF: Weigh 13.1g of lithium carbonate in the reaction kettle, add 32g of pure water, start sti...
Embodiment 2
[0031] Ⅰ.PF 5 Preparation: Weigh 100g of defluorinated slag powder (CaF in defluorinated slag 2 content of 82%), 165g of phosphorus pentoxide in a sealed sample bag, after preliminary mixing, pour it into a laboratory multifunctional pulverizer and pulverize it for about 1min, put the mixture in a tube vacuum furnace, and turn on the vacuum pump to evacuate to 0.06MPa Finally, turn off the vacuum pump, turn on the heating power supply of the tube furnace, turn on the vacuum pump again after the temperature rises to 80°C, and turn off the vacuum pump after the vacuum is evacuated to 0.09MPa. When it reaches 320°C, react for 80 minutes, and the gas generated by the reaction is passed into a closed ether absorption bottle. The mass of ether is 76g, and PF dissolved in 5 125.2g of ether solution;
[0032] Ⅱ. Preparation of LiF: Weigh 16.6g of lithium carbonate in the reaction kettle, add 50g of pure water, start stirring, and at the same time turn on the circulating water in the...
Embodiment 3
[0035] Ⅰ.PF 5 Preparation: Weigh 120g of defluorinated slag powder (CaF in defluorinated slag 2 content of 74%), 220g of phosphorus pentoxide in a sealed sample bag, after preliminary mixing, pour it into a laboratory multifunctional pulverizer and pulverize it for about 1min, put the mixture in a tube vacuum furnace, and turn on the vacuum pump to evacuate to 0.06MPa Finally, turn off the vacuum pump, turn on the heating power supply of the tube furnace, turn on the vacuum pump again after the temperature rises to 80°C, turn off the vacuum pump after evacuating to 0.09MPa, set the temperature of the tube furnace to 280°C (heating rate 5°C / min), and wait until the temperature When it reaches 280°C, react for 60 minutes, and the gas generated by the reaction is passed into a closed ether absorption bottle. The mass of ether is 85g, and PF dissolved in 5 135.3g of ether solution;
[0036] Ⅱ. Preparation of LiF: Weigh 18g of lithium carbonate in the reaction kettle, add 72g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com