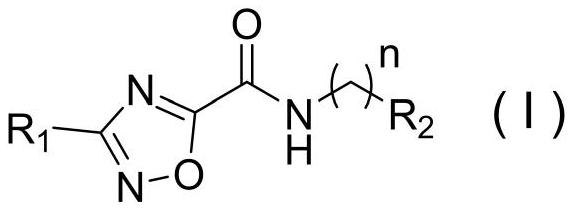
1, 2, 4-oxadiazole-5-formamide derivative as well as preparation method and application thereof
A technology of carboxamides and oxadiazoles, which is applied to 1,2,4-oxadiazole-5-carboxamide derivatives and the fields of preparation and application thereof, can solve the problem of unknown killing effect of plant nematodes and reduce drug consumption. The flexibility of small molecules, the lack of nematicidal activity of pine wood nematodes, etc., achieve the effects of not easy drug resistance, good environmental compatibility, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
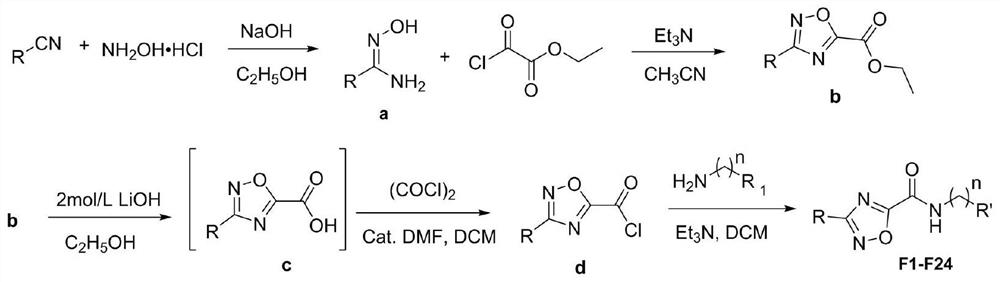
[0072] Embodiment 1: The preparation method of N-(2-(pyridin-2-yl) ethyl)-3-phenyl-1,2,4-oxadiazole-5-carboxamide (compound number is F1), including The following steps:
[0073] (1) Preparation of N-hydroxybenzamide:
[0074] Hydroxylamine hydrochloride (4.04g, 58.18mmol), sodium hydroxide (2.33g, 58.18mmol), and ethanol (48.48mL) were mixed in a 100mL three-necked flask, and substituted formylnitrile (5g, 48.48mmol) was added dropwise at room temperature, and heated after dropping Reflux for 6 hours; after the reaction, the reaction system was washed with water, separated, dried, suction filtered and desolvated under reduced pressure to obtain 5.54 g of N-hydroxybenzamide intermediate, with a yield of 83.92%;
[0075] (2) Preparation of ethyl 3-phenyl-1,2,4-oxadiazole-5-carboxylate:
[0076] Add N-hydroxybenzamide (5g, 36.72mmol), triethylamine (4.45g, 55.08mmol), and acetonitrile (36.72mL) into a three-necked flask, and add monoethyl oxalyl chloride (6.02g, 44.06mmol), c...
Embodiment 2
[0083] Example 2: N-(2-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)ethyl)-3-phenyl-1,2,4-oxadiazole-5-methan The preparation method of amide (compound number is F2), comprises the following steps:
[0084] Steps (1)~(4) are the same as in Example 1;
[0085] (5) The target compound N-(2-(3-chloro-5-(trifluoromethyl)pyridin-2-yl)ethyl)-3-phenyl-1,2,4-oxadiazole-5- Preparation of formamide:
[0086] 2-(3-Chloro-5-(trifluoromethyl)pyridin-2-yl)ethyl-1-amine (0.32 g, 1.44 mmol), triethylamine (0.22 g, 2.16 mmol) and 2.88 mL were dried Add dichloromethane into a one-necked bottle, stir evenly, add 3-phenyl-1,2,4-oxadiazole-5-carbonyl chloride (0.30g, 1.44mmol) at room temperature, and keep the temperature for 9h, After the reaction system was washed with water, washed with saturated sodium bicarbonate, washed with saturated brine, separated, dried, filtered with suction and precipitated under reduced pressure, it was subjected to column chromatography using petroleum ether: ethyl ...
Embodiment 3
[0087] Embodiment 3: The preparation method of N-(2,4-difluorobenzyl)-3-phenyl-1,2,4-oxadiazole-5-carboxamide (compound number is F3), comprising the following steps:
[0088] Steps (1)~(4) are the same as in Example 1;
[0089] (5) Preparation of target compound N-(2,4-difluorobenzyl)-3-phenyl-1,2,4-oxadiazole-5-carboxamide:
[0090] Add 2,4-difluorobenzylamine (0.21g, 1.44mmol), triethylamine (0.22g, 2.16mmol) and 2.88mL of dry dichloromethane into a single-necked flask, stir well, then add 3-benzene Base-1,2,4-oxadiazole-5-carbonyl chloride (0.30g, 1.44mmol), after maintaining the temperature for 9h, the reaction system was washed with water, washed with saturated sodium bicarbonate, washed with saturated brine, separated and dried , suction filtration and desolvation under reduced pressure, using petroleum ether: ethyl acetate = 30:1 as the mobile phase through column chromatography to obtain white solid N-(2,4-difluorobenzyl)-3-phenyl- 0.24 g of 1,2,4-oxadiazole-5-carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com