Symmetric polymer centered on dithienoquinoxaline-containing matrix and flexible electrochromic device
A technology of quinoxaline and dithiophene is applied in the application field of flexible electrochromic devices, which can solve the problems of single color system and monotonous color system, and achieve the effects of enhanced interaction, good planarity and high thermal stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Based on 2,5-dibromo[1,2,5]thiadiazolo[3,4-b]dithieno[2,3-f:3',2'-h]quinoxaline symmetric conjugated Polymer precursor (Structure C). The synthesis reaction process is as follows, and the specific reaction steps and reaction conditions are as follows:
[0048]
[0049] (1) Preparation of Compound A
[0050] In an argon atmosphere at -78°C, a hexane solution of 1.6 moles of n-butyllithium (50 mL, 0.125 mol) was injected into anhydrous tetrahydrofuran (125 mL), and after stirring evenly, 3-bromothiophene (12.7 mL , 0.125mol) was added dropwise thereto to generate 3-lithium thiophene. At the same time, LiBr (10.86 g, 0.125 mol) and CuBr (17.93 g, 0.125 mol) were added into 300 mL of anhydrous tetrahydrofuran at -78° C. to dissolve for later use. Then 4.83mL of oxalyl chloride (7.14g, 56mmol) was dissolved in 100mL of anhydrous tetrahydrofuran and cooled to -78°C for later use. After adding 3-lithium thiophene dropwise into the LiBr / CuBr / THF solution and continuously...
Embodiment 2
[0056] Chemical reactions for the preparation of corresponding polymers by anodic oxidation polymerization based on a symmetrical polymer precursor (structure C, m=3) of dithieno[2,3-f:3',2'-h]quinoxaline The flow process is as follows, and the specific reaction steps and reaction conditions are as follows:
[0057]
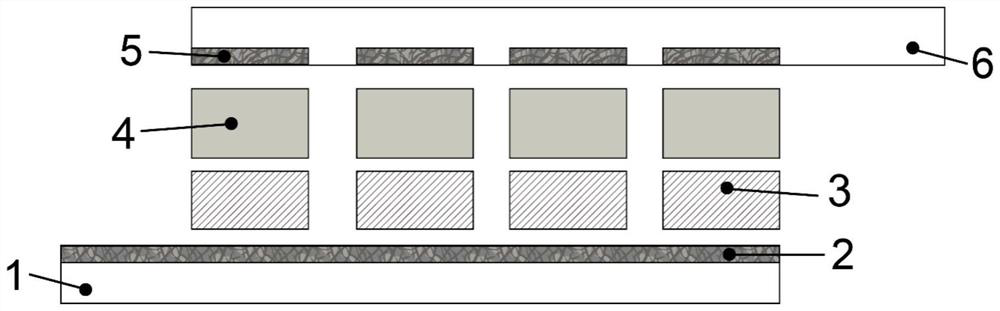
[0058] Under the protection of argon, the symmetrical conjugated polymer precursor (structure C) prepared in Example 1 was dissolved in the refined boron trifluoride ether / dichloromethane (6mL / 24mL) mixed electrolyte, and the concentration 1mmol·L -1 The polymeric precursor (structure C, m=3), 0.1mmol L -1 The tetrabutylammonium phosphorus hexafluoride is used as the electrolyte; the mixed solution is kept in an argon atmosphere, the Cu / PET substrate covered with a mask pattern is used as the working electrode, the platinum sheet is used as the counter electrode, and the Ag / AgCl is used as the reference electrode. Oxidative deposition at a constant potential...
Embodiment 3
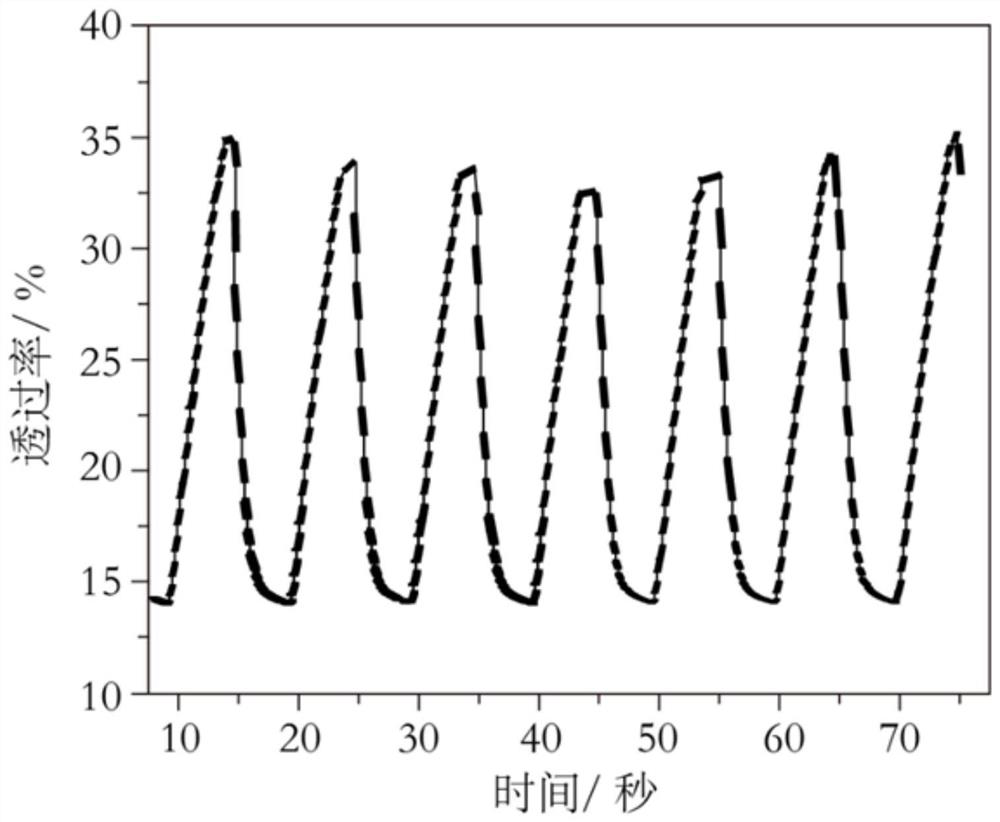
[0060] Taking the polymer material obtained in Example 2 as an example and applying it in the electrochromic field, the following examples will illustrate the symmetrical conjugated polymer provided by the present invention and its application process in the electrochromic field, but the present invention is not limited to Take the example.
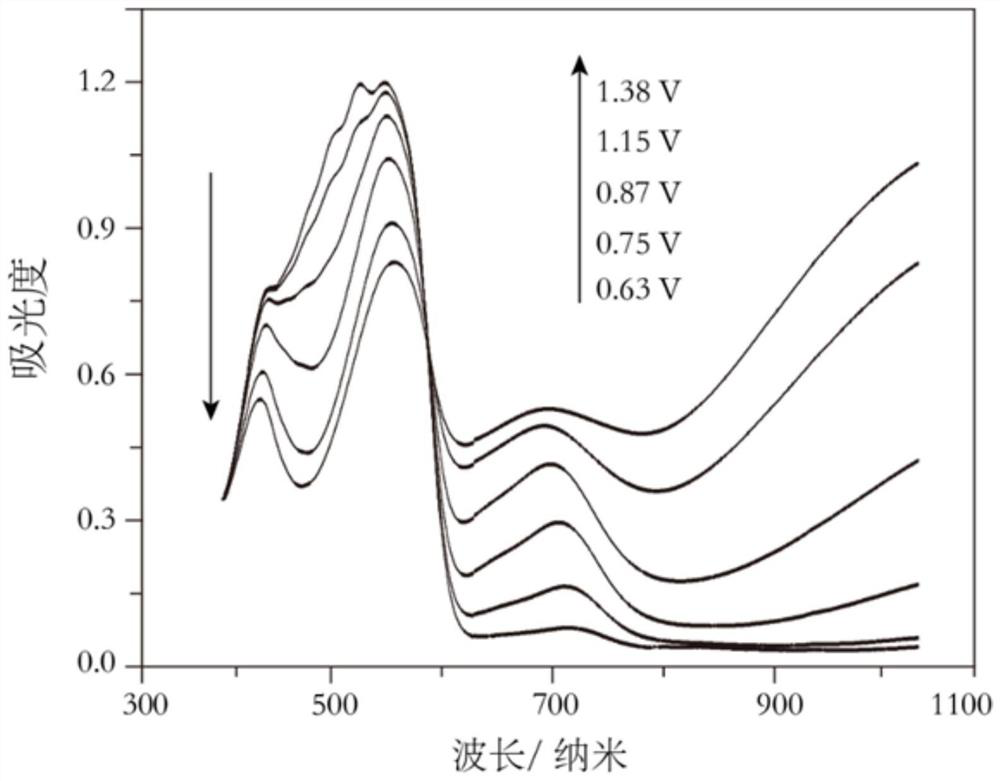
[0061] (1) Electrochemical in situ absorption spectroscopy
[0062] The polymer matrix prepared in Example 2 is used as a working electrode, the platinum sheet is used as a counter electrode, and Ag / AgCl is used as a reference electrode, and placed in a three-electrode electrolytic cell made of quartz, in which 0.5mol L -1 Dichloromethane solution of lithium hexafluorophosphate; use the electrochemical workstation to adjust the voltage applied to the working electrode, and at the same time record the evolution of the absorption spectrum of the polymer electrode at different voltages with a UV-visible spectrophotometer to obtain the electr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com