Methionine hydroxyl analogue isopropyl ester compound as well as preparation method and production system thereof
A technology of methionine hydroxyl and analogues, which is applied in the fields of sulfide preparation, animal feed, food science, etc., can solve the problems of waste of resources, high energy consumption of products and residual liquid treatment, and high boiling point of esterification products, so as to reduce carbon emissions and Effects of sulfur oxide emission, saving incineration treatment process, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
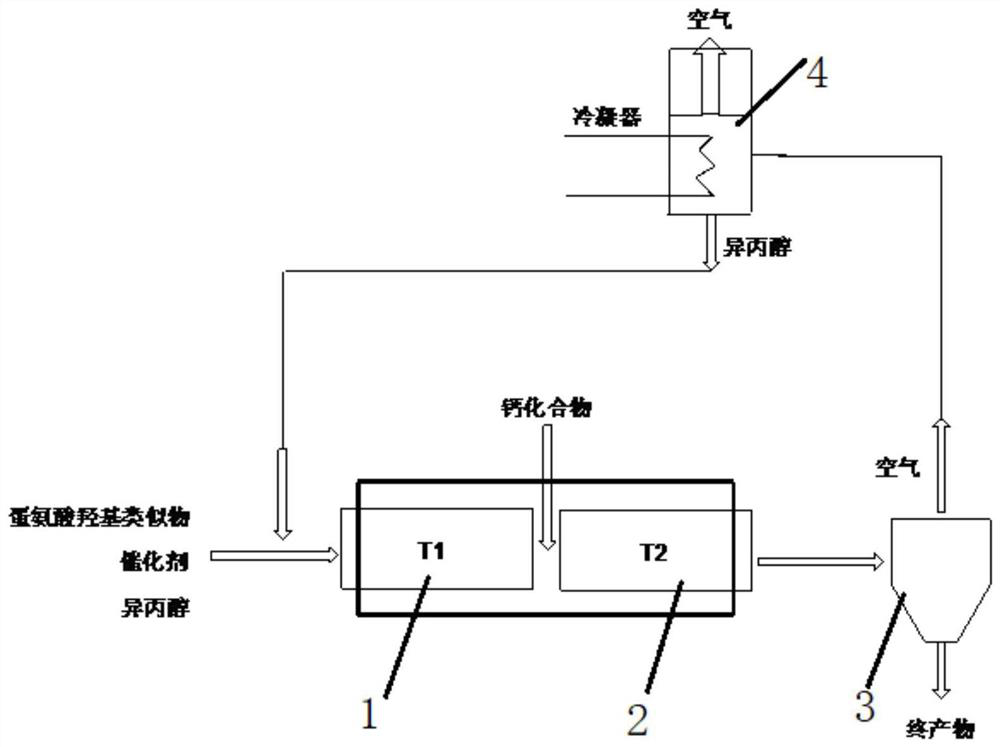
Image
Examples
Embodiment 1
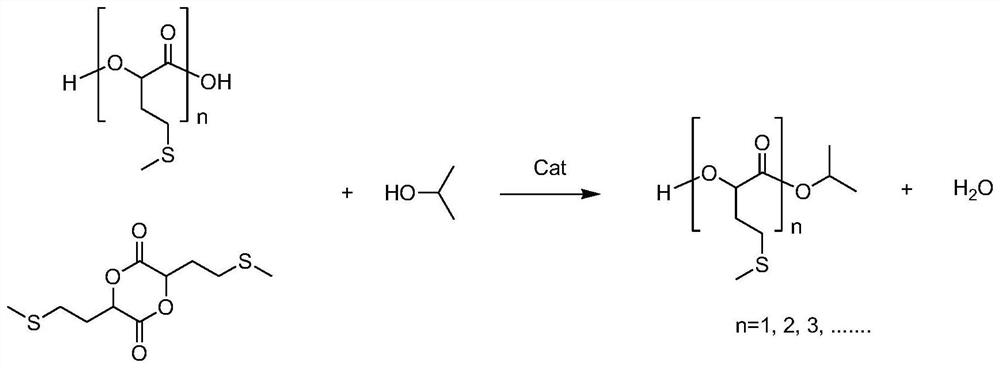
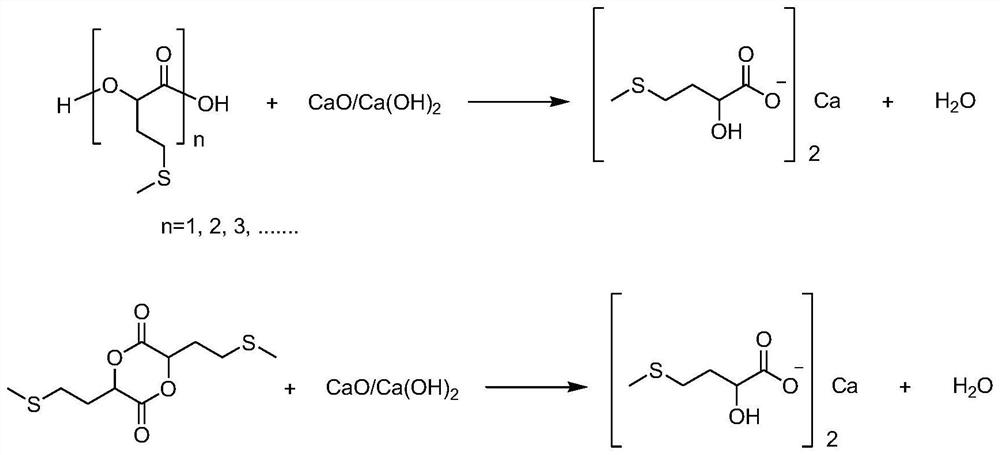
[0047] 151.15 g (1.0 mol) of methionine methionine hydroxy analogue with a mass percentage of 99%, 150.76 g (1.0 mol) of phosphoric acid with a mass percentage of 65% and a mass percentage of 99% were added to a 1000 ml reaction flask. 484.85 g (8.0 mol) of isopropanol of 484.85 g (8.0 mol) were stirred and heated to 110° C., maintained in a reflux state, and reacted for 2.5 hours. During the reaction, high-performance liquid chromatography analysis was performed to monitor the increase of methionine hydroxyl analog isopropyl ester in the reaction process. After the reaction was completed, the obtained mixed solution was cooled to 45°C, and under stirring, 38.54 grams of calcium hydroxide powder with a mass percentage of 96% was slowly added, and the neutralization reaction temperature was controlled at 40°C to 45°C. The endpoint pH value of the reaction was controlled at 3.5, then directly concentrated under reduced pressure to a solvent-free state, the obtained solid was tran...
Embodiment 2
[0049] 170.45 g (1.0 mol) of methionine methionine hydroxy analogue with a mass percentage of 88%, 46.12 g (0.4 mol) of phosphoric acid with a mass percentage of 85% and a mass percentage of 99% were added to a 1000 ml reaction flask. 303.54 g (5.0 mol) of isopropanol was stirred to be warmed up to 85° C., kept a slightly boiling state, and reacted for 3 hours. In the reaction process, high-performance liquid chromatography analysis monitored the increase of methionine hydroxyl analog isopropyl ester in the reaction process. After the reaction was completed, the obtained mixed solution was cooled to 40°C, and 22.85 grams of calcium oxide powder was slowly added under stirring, the neutralization reaction temperature was controlled at 40°C to 45°C, and the endpoint pH value of the neutralization reaction was controlled at 6.0. Then directly decompressed and concentrated to a solvent-free state, the obtained solid was transferred to an enamel tray, put into a blast drying oven an...
Embodiment 3
[0051]157.89 g (1.0 mol) of methionine methionine hydroxy analogue with a mass percentage of 95%, 226.15 g (1.5 mol) of phosphoric acid with a mass percentage of 65% and a mass percentage of 99% were added to a 1000 ml reaction flask. 484.85 g (5.0 mol) of isopropanol was stirred and warmed up to 100° C., maintained in a reflux state, and reacted for 3.0 hours. During the reaction, high-performance liquid chromatography was used to analyze and monitor the increase of methionine hydroxyl analog isopropyl ester in the reaction process. After the reaction is completed, the obtained mixed solution is cooled to 45°C, and under stirring, 118.5 grams of calcium hydroxide powder with a mass percentage of 96% is slowly added, and the neutralization reaction temperature is controlled at 40°C to 45°C. The terminal pH value of the reaction was controlled at 6.5, then directly concentrated under reduced pressure to a solvent-free state, the obtained solid was transferred into an enamel tray...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com