Controlled vapor deposition of biocompatible coatings for medical devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example one
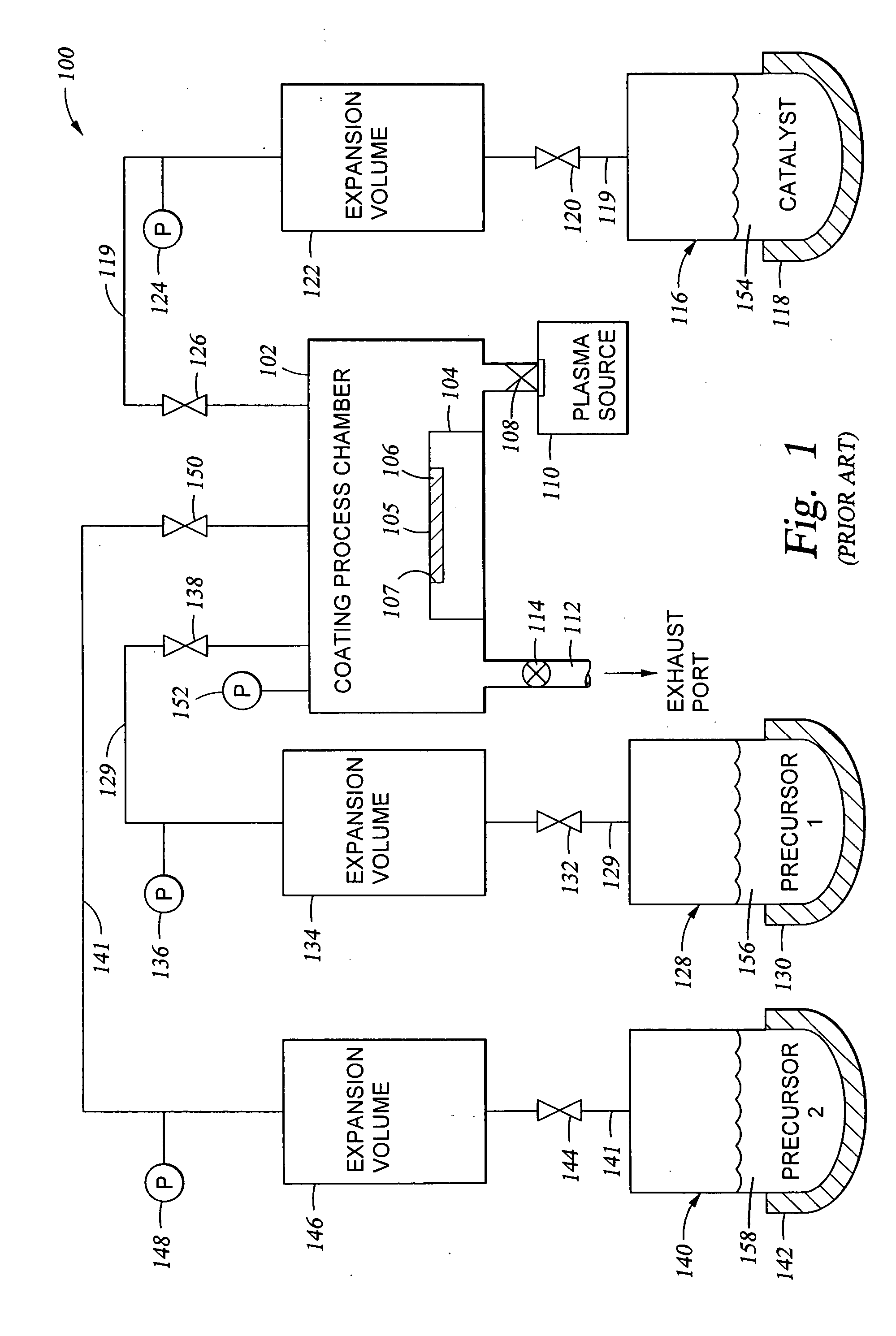
[0112] The vapor deposition techniques described previously herein were used to coat devices such as implantable (intraocular) lenses with a hydrophilic oxide / polyethylene glycol coating. Prior to deposition of the coating, the device surface was pre-treated by exposure to an oxygen plasma (150-200 sccm O2 at an RF power of about 200 W and a process chamber pressure of 0.3 Torr in an Applied MicroStructures' MVD™ process chamber) for 5 minutes in order to clean the surface and create hydroxyl availability on a substrate surface (by way of example and not by way of limitation).
[0113] Following oxygen plasma treatment of the lens, SiCl4 was charged to the process chamber from a SiCl4 vapor reservoir, where the SiCl4 vapor pressure in the vapor reservoir was 18 Torr, creating a partial pressure of 2.3 Torr in the coating process chamber. Within 5 seconds, a first volume of H2O vapor was charged to the process chamber from a H2O vapor reservoir, where the H2O vapor pressure in the vapo...
example two
[0148] Deposition of a Silicon Oxide Layer Having a Controlled Number of OH
[0149] Reactive Sites Available On the Oxide Layer Surface
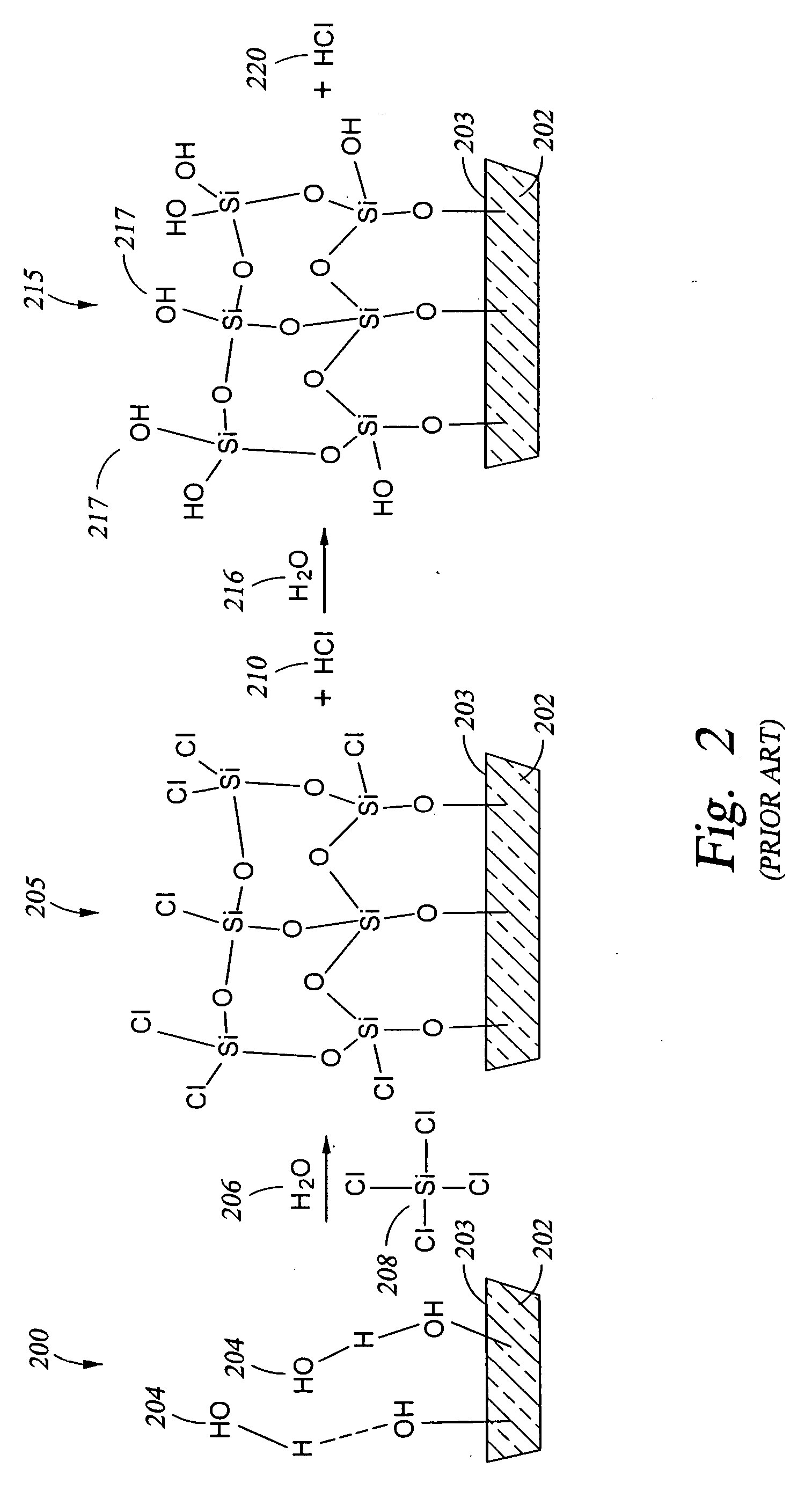
[0150] A technique for adjusting the hydrophobicity / hydrophilicity of a substrate surface (so that the surface is converted from hydrophobic to hydrophilic or so that a hydrophilic surface is made more hydrophilic, for example) may also be viewed as adjusting the number of OH reactive sites available on the surface of the substrate. One such technique is to apply an oxide coating over the substrate surface while providing the desired concentration of OH reactive sites available on the oxide surface. A schematic 200 of the mechanism of oxide formation in shown in FIG. 2. In particular, a substrate 202 has OH groups 204 present on the substrate surface 203. A chlorosilane 208, such as the tetrachlorosilane shown, and water 206 are reacted with the OH groups 204, either simultaneously or in sequence, to produce the oxide layer 205 shown on surface 203 o...
example three
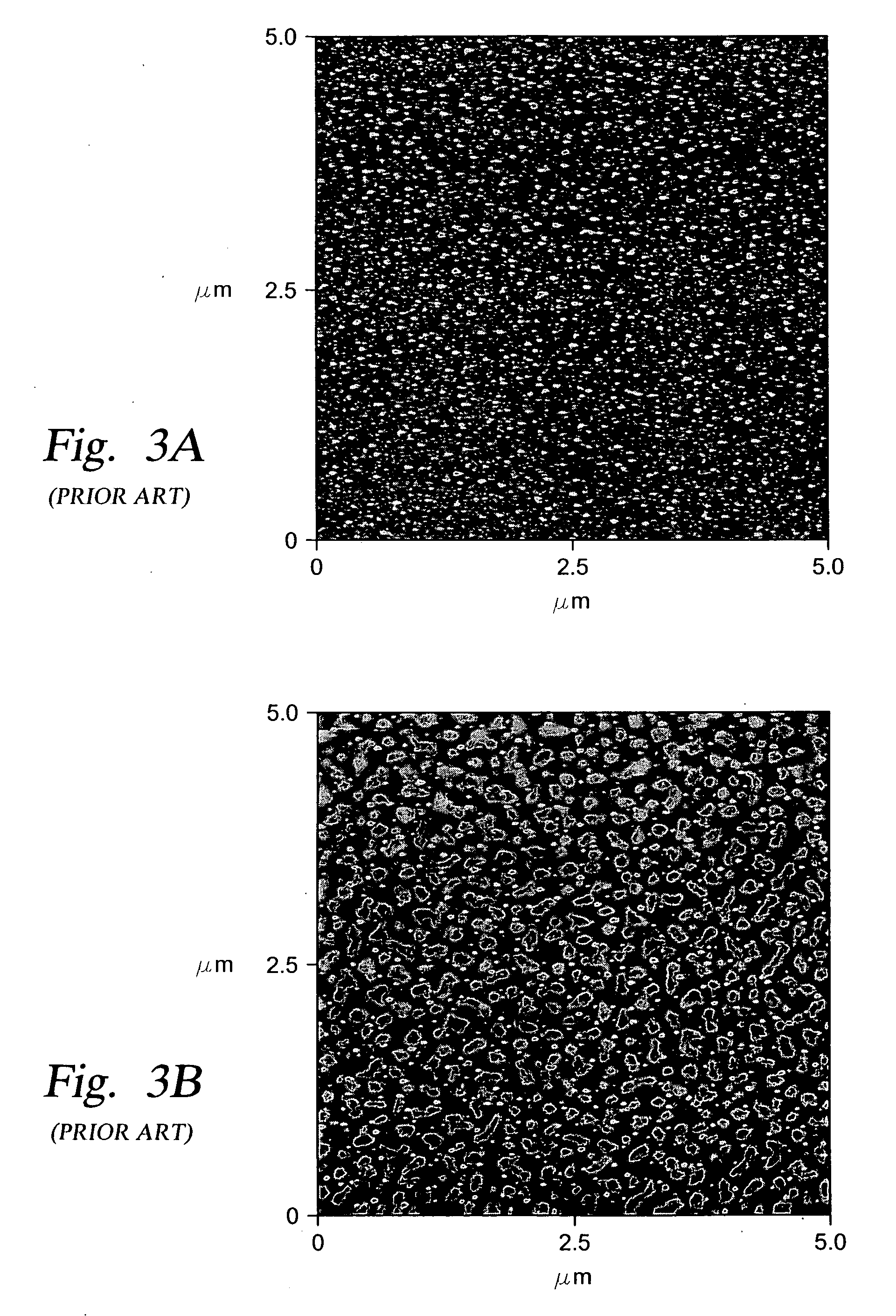
[0152] In the preferred embodiment discussed below, the silicon oxide coating was applied over a glass substrate. The glass substrate was treated with an oxygen plasma in the presence of residual moisture which was present in the process chamber (after pump down of the chamber to about 20 mTorr) to provide a clean surface (free from organic contaminants) and to provide the initial OH groups on the glass surface.
[0153] Various process conditions for the subsequent reaction of the OH groups on the glass surface with vaporous tetrachlorosilane and water are provided below in Table 2, along with data related to the thickness and roughness of the oxide coating obtained and the contact angle (indicating hydrophobicity / hydrophilicity) obtained under the respective process conditions. A lower contact angle indicates increased hydrophilicity and an increase in the number of available OH groups on the silicon oxide surface.
TABLE 2Deposition of a Silicon Oxide Layer of Varying Hydrophilicit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com