In Situ Remediation of Inorganic Contaminants Using Stabilized Zero-Valent Iron Nanoparticles
a zero-valent iron nanoparticle, in situ technology, applied in water treatment compounds, water/sludge/sewage treatment, chemical instruments and processes, etc., can solve the problems of contamination legacy, inability to use in situ applications, rapid loss of soil mobility and reactivity, etc., and achieve low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
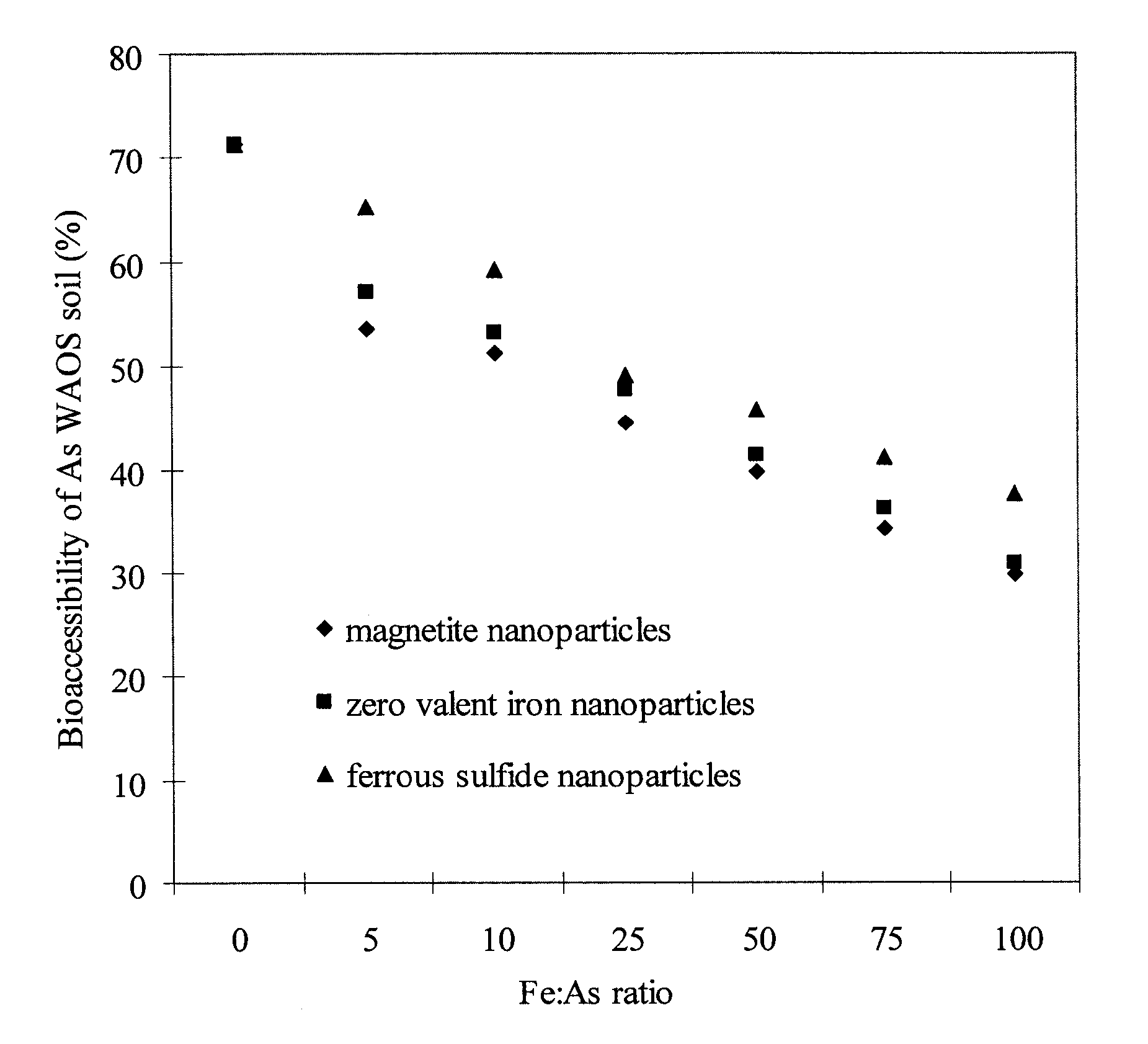
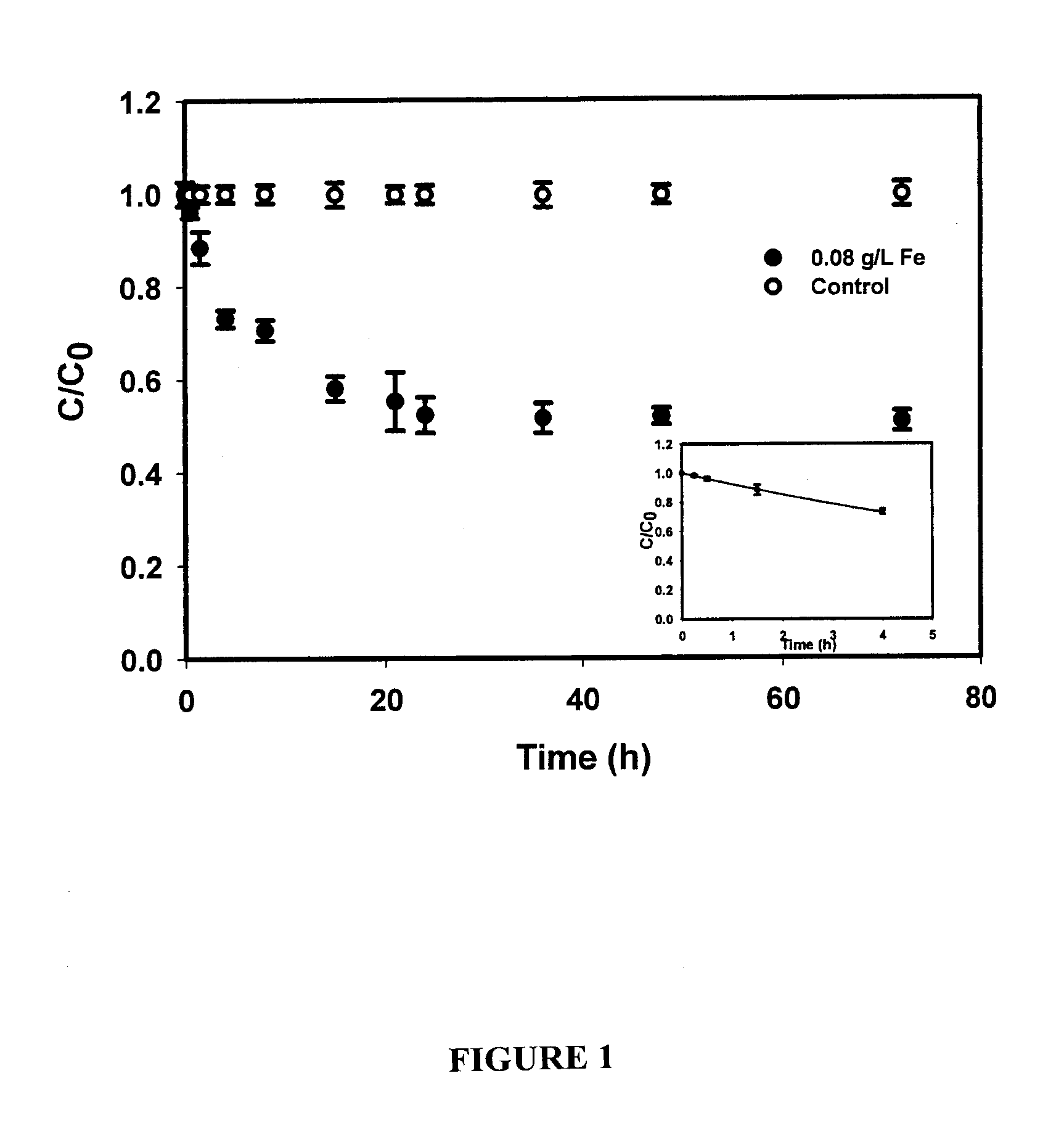
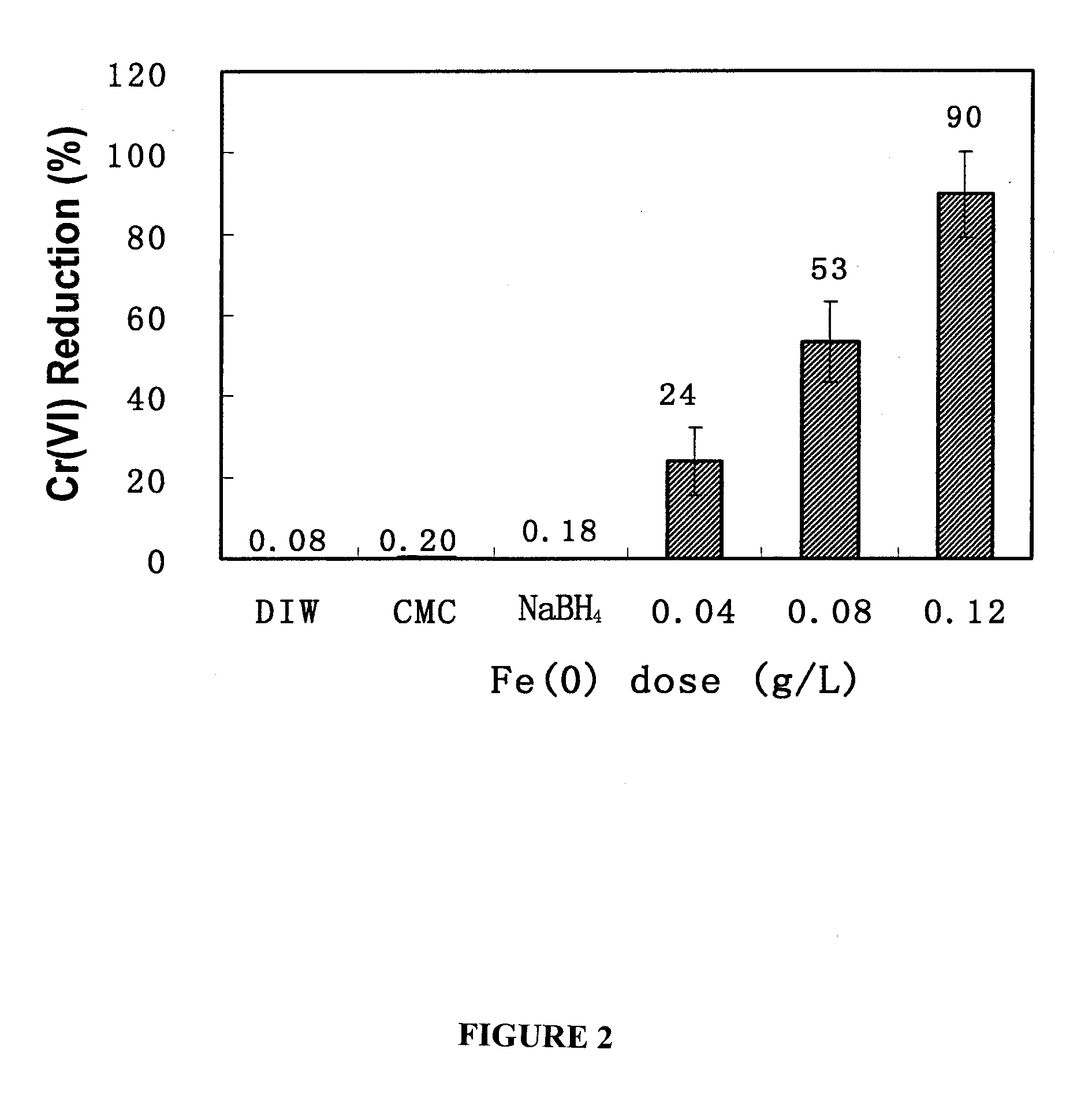
[0093] The overall goal of this experiment was to test the feasibility of using the CMC-stabilized ZVI nanoparticles developed by He et al. (2006) for in situ reductive immobilization of Cr(VI) in contaminated soils. The specific objectives of this work were to: (1) test the CMC-stabilized nanoparticles for reducing and removing Cr(VI) in water and in a sandy loam soil slurry under various experimental conditions, and (2) test the stabilized nanoparticles for reductive immobilization of Cr(VI) in a contaminated sandy loam through fixed-bed column elution experiments.
1. Materials and Methods
[0094] Chemicals of analytical grade or higher were used in this research, including iron(II) sulfate heptahydrate (FeSO4.7H20, Acros Organics, Morris Plains, N.J., USA), sodium borohydride (NaBH4, ICN Biomedicals, Aurora, Ohio., USA), sodium carboxymethyl cellulose (CMC, Acros Organics, Morris Plains, N.J., USA), sodium chromate tetrahydrate (Na2Cr04.4H2O, Aldrich, Milwaukee, Wis., USA), and 1...
example 2
[0126] This present experiment aims to test the feasibility of using the CMC-or starch-stabilized ZVI nanoparticles for perchlorate destruction in fresh water or in typical spent IX regenerant brine or contaminated saline water. The specific objectives are to: 1) determine the rate and extent of perchlorate reduction by stabilized ZVI nanoparticles; and 2) characterize the influences of temperature, salinity, and pH on the reaction rate.
1. Materials and Methods
1.1. Chemicals
[0127] The following chemicals were used as received: 4-(2-Hydroxyethyl)-1-piperazineethane ethanesulfonic acid (HEPES, C8H18N2O4S) (Fisher, Fair Lawn, N.J., USA); aluminum chloride (AlCl3.6H2O) (Fisher); cobalt chloride (CoCl2.6H2O) (Fisher); cupric chloride (CuCl2.2H2O) (Fisher); ferrous sulfate (FeSO4.7H2O) (Acros Organics, Morris Plains, N.J., USA); methyltrioxorhenium (VII) (MeReO3, 98%) (Strem Chemicals, Newburyport, Mass., USA); nickel chloride (NiCl2.6H2O) (Fisher); potassium hexachloropalladate (K2P...
example 3
[0163] The overall goal of this experiment is to test the effectiveness of using the CMC-stabilized Fe0 nanoparticles for rapid degradation of nitrate in water. The specific ojectives are to: 1) prepare CMC-stabilized Fe0 nanoparticles following the approach by He and Zhao (2005); 2) determine the rate and extent of nitrate reduction by the stabilized Fe0 nanoparticles; 3) characterize the influences of pH, salinity and a metal catalyst on the nitrate reaction rate; and 4) test the effectiveness of using stabilized Fe0 for degradation of nitrate in saline water.
1. Materials and Methods
1.1. Chemicals.
[0164] The following chemicals (analytical grade) were used as received: 4-(2-Hydroxyethyl)-1-Piperazineethaneethanesulfonic acid (HEPES, C8H18N2O4S, Fisher, Fair Lawn, N.J.); 4-Morpholinoethanesulfonic acid (MES, C6H13NO4S.xH2O, Fisher, Fair Lawn, N.J.); Ammonium nitrate (NH4NO3, Fisher, Fair Lawn, N.J.); Iron (II) sulfate (FeSO4.7H2O, Acros Organics, Morris Plains, N.J.); Nessler ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com