Method of removing metals from hydrocarbon feedstock using esters of carboxylic acids
a carboxylic acid and hydrocarbon feedstock technology, applied in the field of hydrocarbon industry, can solve problems such as heat exchanger fouling, catalyst poisoning, and effluent treatment difficulties, and achieve the effect of increasing efficiency and non-corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Test Method and Results for Use of Additives
[0083]Procedure: The inventor of present invention has used the following invention additive for calcium-removal.
1.Diethyl Maleate2.Dimethyl Maleate3.Dibutyl Maleate4.Methyl Formate5.Ethyl Formate6.Ethyl Acetate7.Dimethyl Fumerate8.Diethyl Oxalate #9.Formic Acid 98%10.Di Octyl Maleate11.Acrylic Acid12.Methyl Acrylate13.Methyl Methacrylate14.Dimethyl Succinate15.Diethyl succinate16.Maleic Anhydride + Methanol + Water17.Maleic Anhydride + Methanol18.Maleic Anhydride + Isopropyl alcohol19.Maleic Anhydride + Ethanol20.Maleic Anhydride +Sodium hydroxide + Water
[0084]Each additive of the present invention, demineralized water and Ca-naphthenate in toluene was charged into a stainless steel autoclave and was reacted at different reaction conditions given below.
TABLE 3TemperatureTime of reactionTable Nos1130° C.20 minutes102130° C.10 minutes113130° C. 1 minute124115° C.15 minutes135115° C. 1 minute14
[0085]It was cooled to room temperature and the...
example 2
Preparation of Methanolic Solution of Additive of Present Invention
[0087]In the preparation of methanolic solution of the additive of the present invention, the following steps were used:[0088](a) 30 gm of methanol was charged to a clean four-necked round bottom flask, equipped with thermometer, stirrer, and inlet for nitrogen,[0089](b) Total of 33 gm of maleic anhydride was added into the above mentioned flask, in six lots;[0090](c) The mixture was stirred well till a clear solution was obtained, thereby indicating completion of formation of maleic ester;[0091](d) 37 gms of water was added to the clear solution;[0092](e) The exotherm of approximately 5° C. to 10° C. was noted;[0093](f) The mixture was mixed well;[0094](g) The mixture was analyzed for acid value which was found to be 225 mg KOH / gm; it was observed that the acid value drops on storage of the mixture. For example, the acid value was 196 mg KOH / gm after storing for 17 days and was 145 mg KOH / gm after storing for one ye...
example 3
[0100]Reaction of Maleic Anhydride with Methanol
TABLE 4Mole ratio of Maleic anhydride with Methanol is 1:1.25WtMolecularcharged inProduct NamewtMolegms% wtMaleic981.09871.014anhydrideMethanol321.254028.986Total # size138100
Procedure:
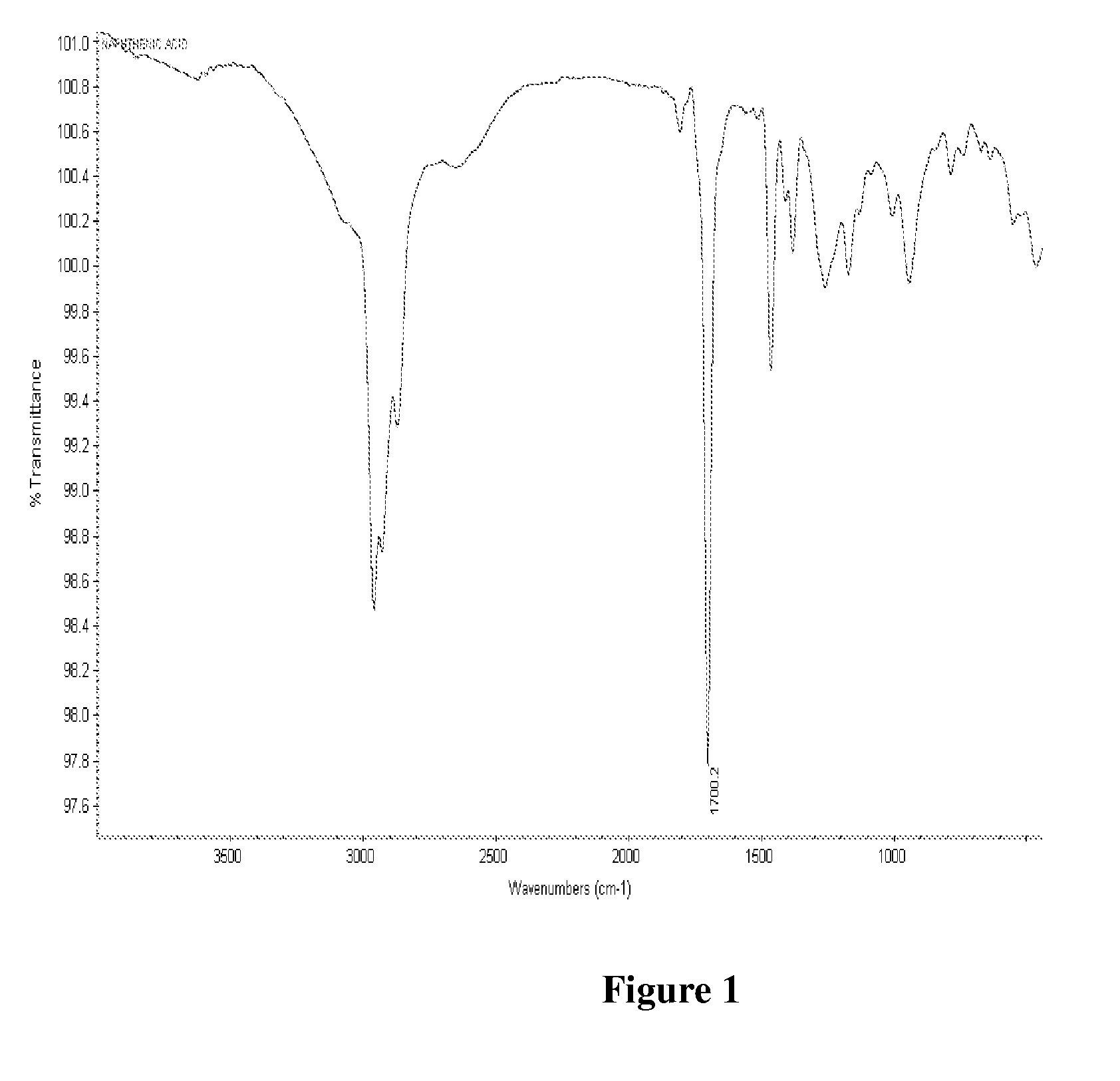
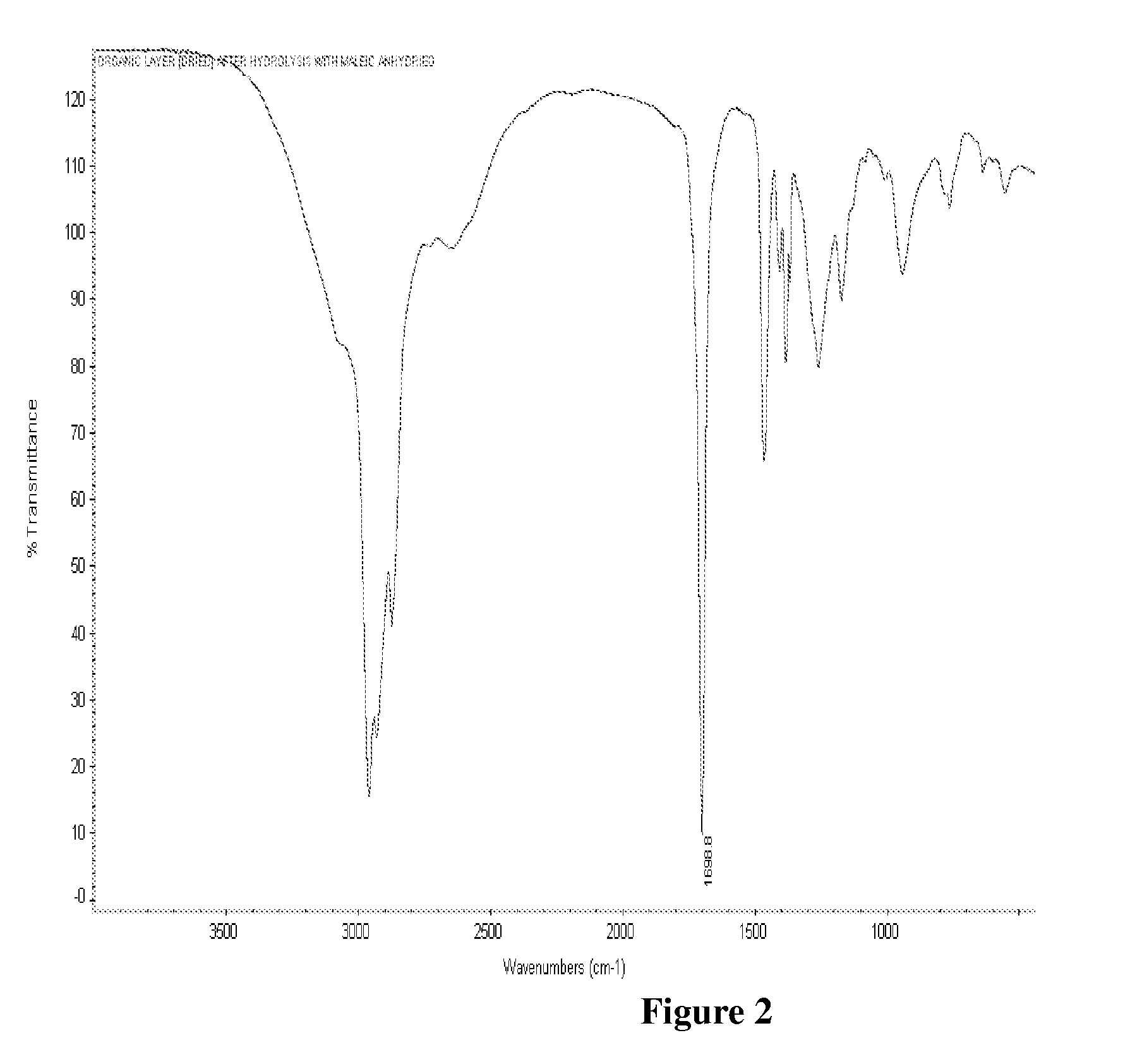
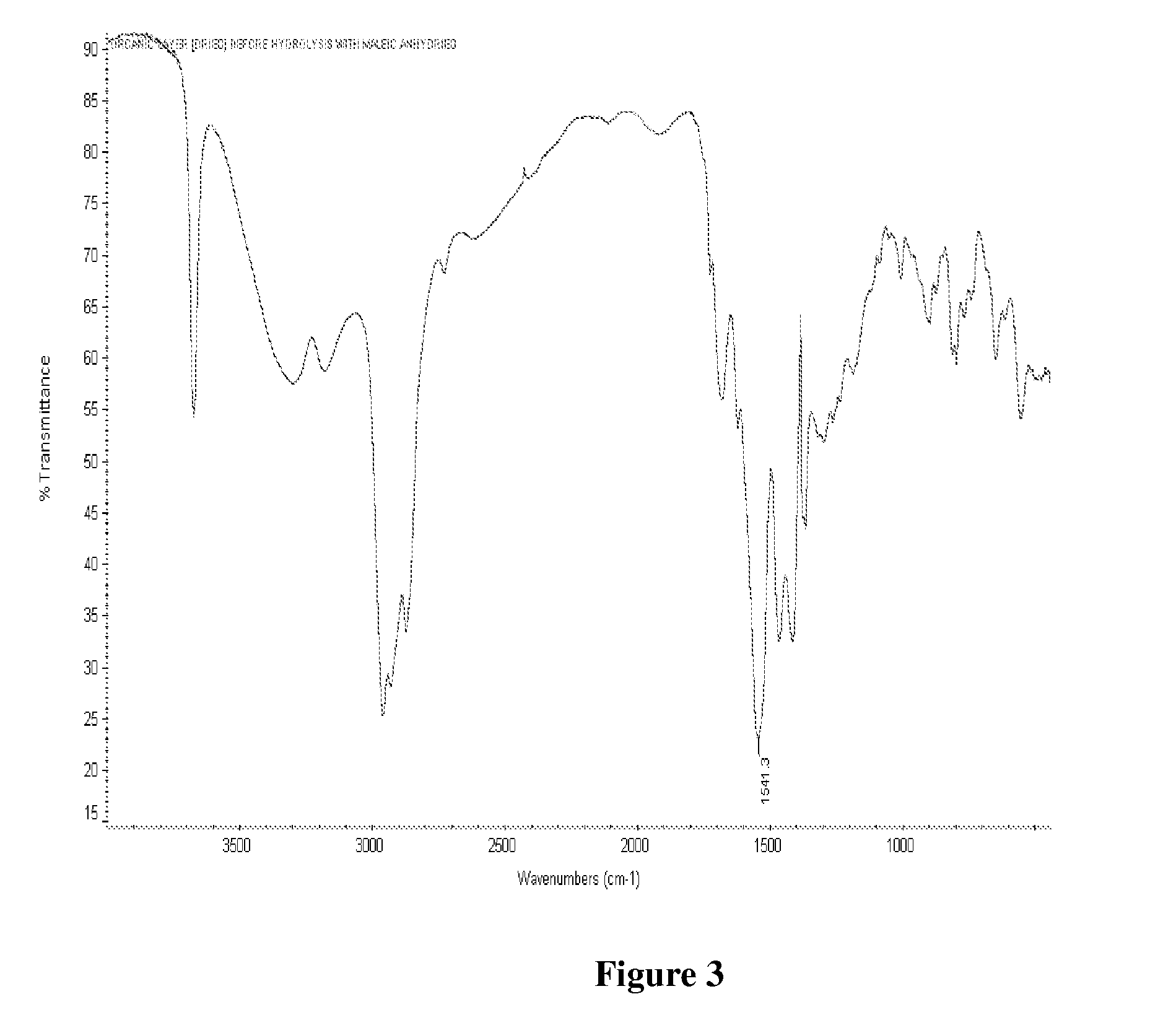
[0101]One mole of Maleic anhydride was charged to a clean 250 ml 4 neck RBF equipped with stirrer rod with Teflon blade, Thermometer pocket, water condenser, a dropping funnel and a stopper. The charged compound was heated to 55 deg C. and then 1.25 moles of methanol was added dropwise. During the addition of methanol exotherm was observed. After completion of methanol addition temperature was slowly raised to 80° C. and maintained for 2 hours. At the end of this period, the reaction mass was cooled to room temperature that is about 27° C. The reaction mixture was analysed for Acid Value by titrating against potassium hydroxide. Also a small portion of the sample was dried and analysed by FTIR. The FTIR showed the presence of peak at 1735 cm−1 indicating...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact time | aaaaa | aaaaa |
| contact time | aaaaa | aaaaa |
| contact time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com