Chinese traditional extraction and the function containing dammarane type four-ring triterpene sapogenin
A tetracyclic triterpenoid, dammarane type technology, applied in the field of dammarane type tetracyclic triterpenoid saponin extract, can solve the problems of protein function damage, cell energy metabolism disorder, cell function damage, etc. Cardiotoxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Take 500g of Panax notoginseng medicinal material granules (3mm-8mm), dry at 50°C for 6 hours, add 10 times the amount of 70% ethanol and heat and reflux in a slightly boiling state to extract twice, each time for 2 hours, filter the extract, combine, and concentrate under reduced pressure to 0.5 g / mL (crude drug amount), above D 101 Macroporous resin. Rinse with 4 times the column volume of deionized water, discard, then rinse with 4BV of 0.05% ammonia water, discard, then rinse with deionized water until the effluent pH=7, continue to rinse with 4BV of 30% ethanol solution, discard, Finally, it was eluted with 5BV 70% ethanol solution, and the eluate was collected, concentrated under reduced pressure to no alcohol, and freeze-dried to obtain the total saponins of Panax notoginseng. Determined by HPLC external standard method, notoginseng saponin R in the refined product 1 8%, Ginsenoside Rg 1 44%, Ginsenoside Rb 1 28%, ginsenoside Rd10%, ginsenoside Re 5%, the ...
Embodiment 2
[0018] The notoginseng medicinal material is crushed to the size of soybeans to coarse powder, extracted 4 times with 40% water-containing alcohol under reflux, recovered the solvent, extracted 3 times with an equal volume of n-butanol, combined the extracts, concentrated to dryness, crushed, and washed with petroleum ether for 2 times, desolvation and drying. Adopt high performance liquid phase method (HPLC) to detect it, analysis condition is Agilent Extend-C18, mobile phase H 2 O-CH3CN, the detection wavelength is 203nm, and its content is determined by the external standard method. The contents of the five components are respectively notoginseng saponin R 1 3%, Ginsenoside Rg 1 25%, Ginsenoside Rb 1 14%, ginsenoside Rd 4%, ginsenoside Re 1.5%. The total content is 47.5%.
Embodiment 3
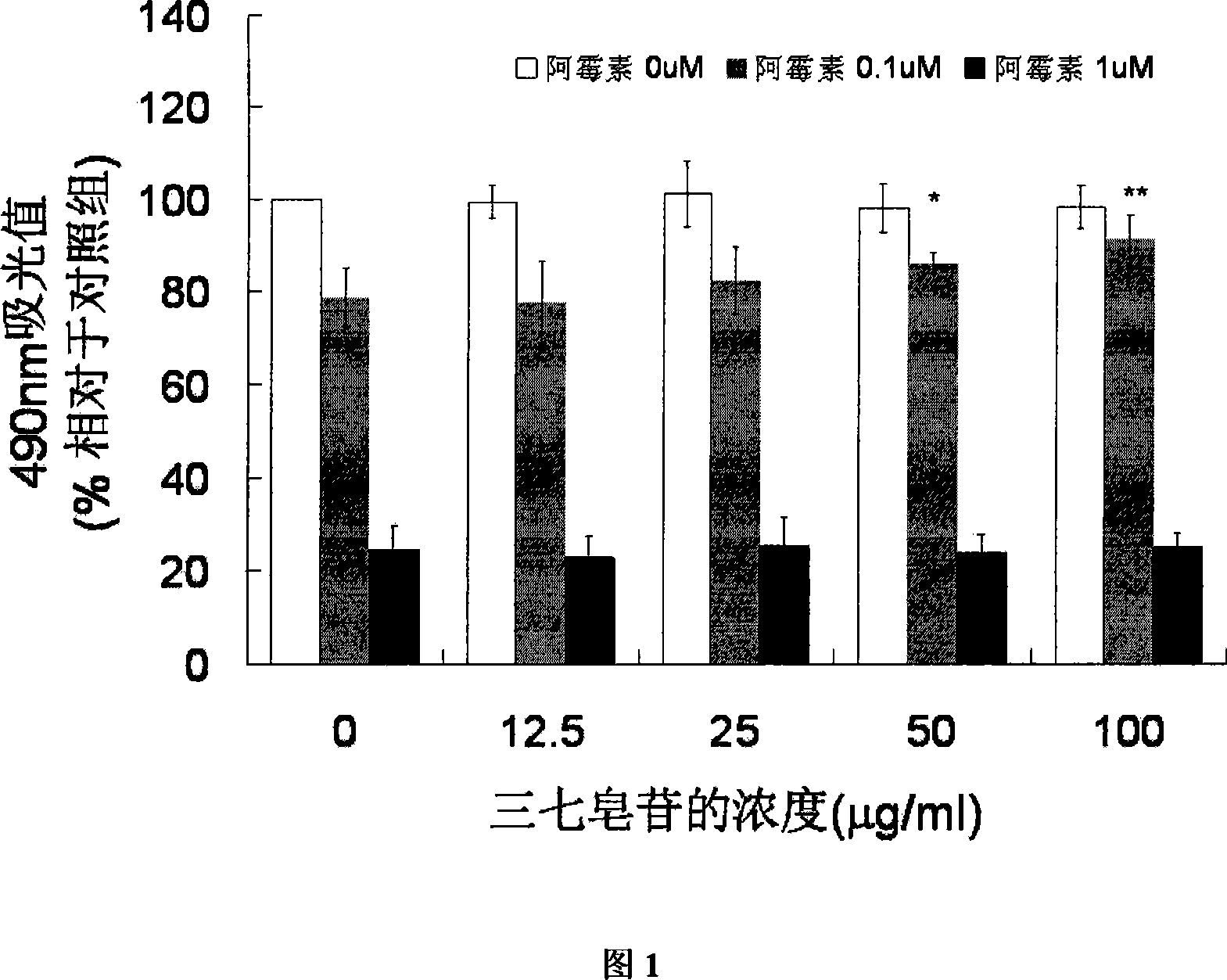
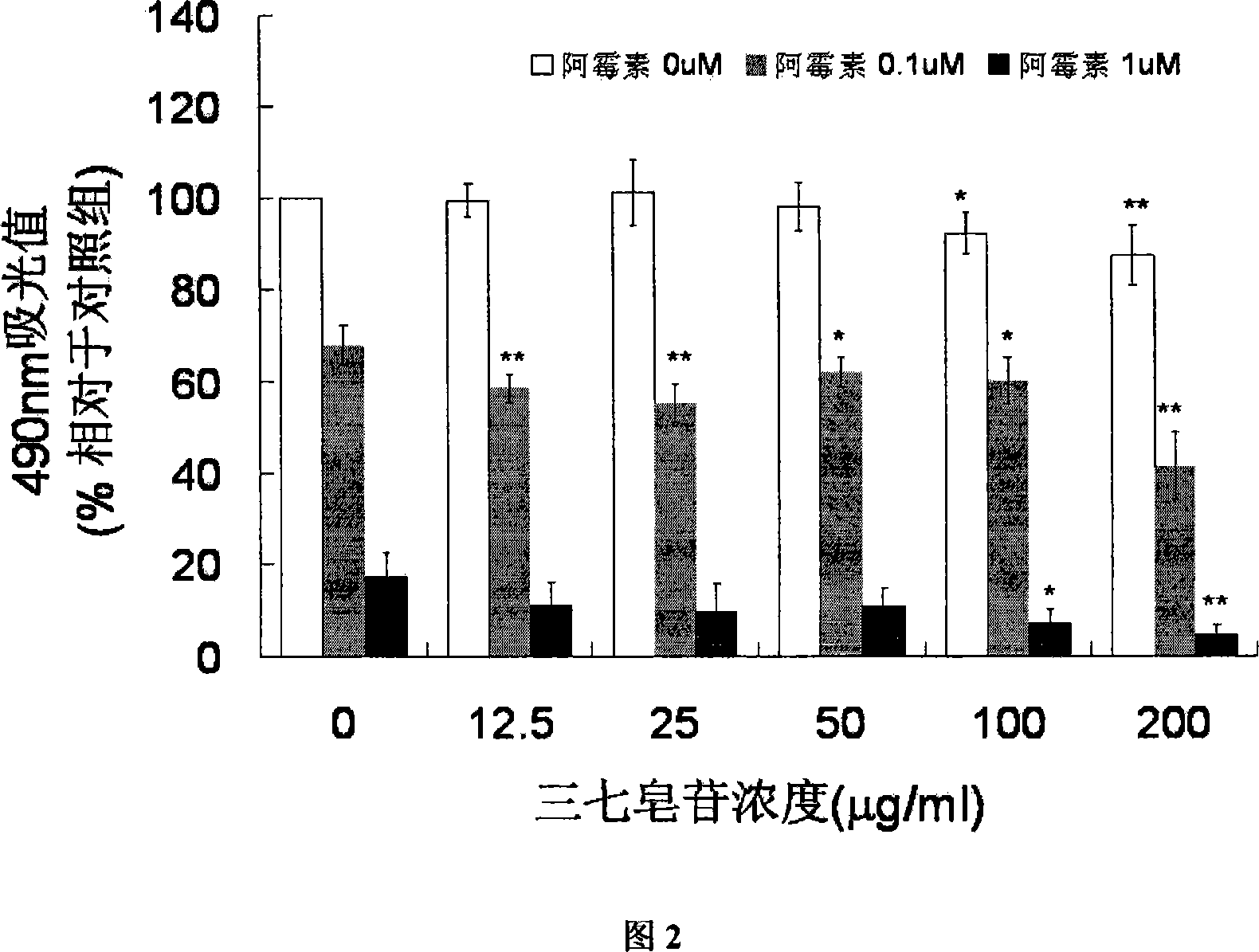
[0020] Cardiomyocytes from neonatal rats (Wistar rats) were isolated and seeded into 96-well plates. Cell density 5×10 5 pcs / hole. After culturing in DMEM+20% FBS medium for 48 hours, the cells adhered to the wall normally, and some cells beat regularly. Set up control group, model group (containing 0.1, 1 μ M doxorubicin hydrochloride), and different concentrations (12.5-100 μ g / ml) of notoginseng saponin group (added 1 hour before adding doxorubicin). After culturing for 24 hours, it was measured by MTT method, and the viability of cardiomyocytes was reflected by the 490nm colorimetric value. The results are shown in Figure 1 ( ** P* P<0.05).
[0021] Experiments have shown that 0.1 and 1 μM doxorubicin hydrochloride can cause significant myocardial cell damage, and the MTT value is significantly reduced; while the pre-administration of notoginseng saponin at a concentration of 12.5-100 μg / ml shows a certain protective effect. It shows that notoginseng saponins can prev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com