Method for making ferrous lithium phosphate/carbon compound material of high active disorderly ferric phosphate
A technology of lithium ferrous phosphate and carbon composite materials, which is applied in the fields of phosphorus compounds, chemical instruments and methods, and inorganic chemistry. Capacity, the effect of avoiding product impurity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of ferric phosphate: mix the ferrous phosphate with a concentration of 0.1 mol / L and phosphoric acid solution in equal volume, add hydrogen peroxide solution with a mass concentration of 20% under stirring, adjust the pH to 3, and after 12 hours of reaction, suction filter , Washing, drying to obtain highly active disordered iron phosphate (FePO 4 ·2H 2 O).
[0025] Weigh iron phosphate (FePO 4 ·2H 2 O) 33.66g, lithium hydroxide (LiOH·H 2 O) 7.938g, 2.844g anhydrous glucose, add ethanol, ball mill at 400r / min for 10 hours, spray dry, program the temperature in a nitrogen atmosphere, increase to 550°C at 5°C / min, keep it warm for 12h, natural After cooling, a lithium iron phosphate / carbon composite material is obtained.
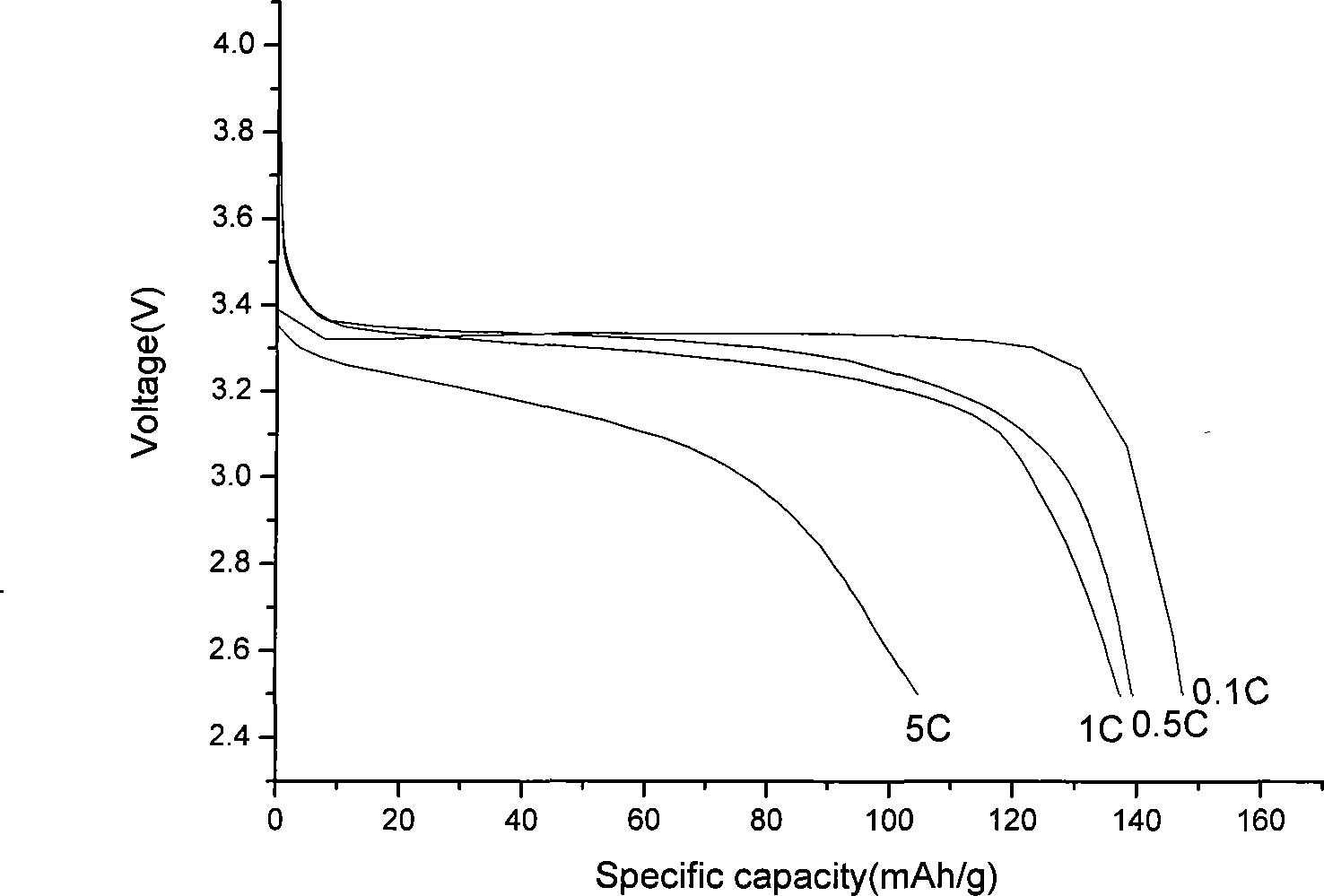
[0026] At room temperature, with a lithium sheet as the negative electrode, the discharge capacity curve of the lithium iron phosphate / carbon composite material at various rates is shown in the accompanying drawings.
Embodiment 2
[0028] The preparation of iron phosphate is the same as in Example 1. Combine 1mol phenol with excess formaldehyde in NH 4 Under OH catalysis, react at 50-90°C for 10 hours, then adjust the pH value to neutral with hydrochloric acid, and continue to react for 5 hours to obtain a resol phenolic resin. Weigh iron phosphate (FePO 4 ·2H 2 O) 33.66g, lithium hydroxide (LiOH·H 2O) 7.56g, 4.266g phenolic resin, add anhydrous ethanol, ball mill at 400r / min for 10 hours, spray dry, program temperature rise in nitrogen atmosphere, increase to 700°C at 5°C / min, keep it warm for 8 hours , Natural cooling, to obtain lithium iron phosphate / carbon composite material. With a lithium sheet as the negative electrode, the discharge capacity of the lithium iron phosphate / carbon composite material can reach 138mAh / g at a rate of 1C.
Embodiment 3
[0030] Preparation of iron phosphate: mix 0.1mol / L ferrous sulfate and ammonium monohydrogen phosphate solution in equal volume, add 15% hydrogen peroxide solution with mass concentration under stirring, adjust the pH to 4, and react for 18 hours , Suction filtration, washing, drying to obtain highly active disordered iron phosphate (FePO 4 ·2H 2 O).
[0031] Weigh iron phosphate (FePO 4 ·2H 2 O) 33.66g, lithium carbonate (Li 2 CO 3 ) 13.32g, super P2.2752g, add distilled water, ball mill at 400r / min for 10 hours, spray dry, program temperature rise in nitrogen atmosphere, increase to 450°C at 5°C / min, keep for 12 hours, cool naturally , To obtain lithium iron phosphate / carbon composite material. With a lithium sheet as a negative electrode, the discharge capacity of the lithium iron phosphate / carbon composite material can reach 132 mAh / g at a rate of 1C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com