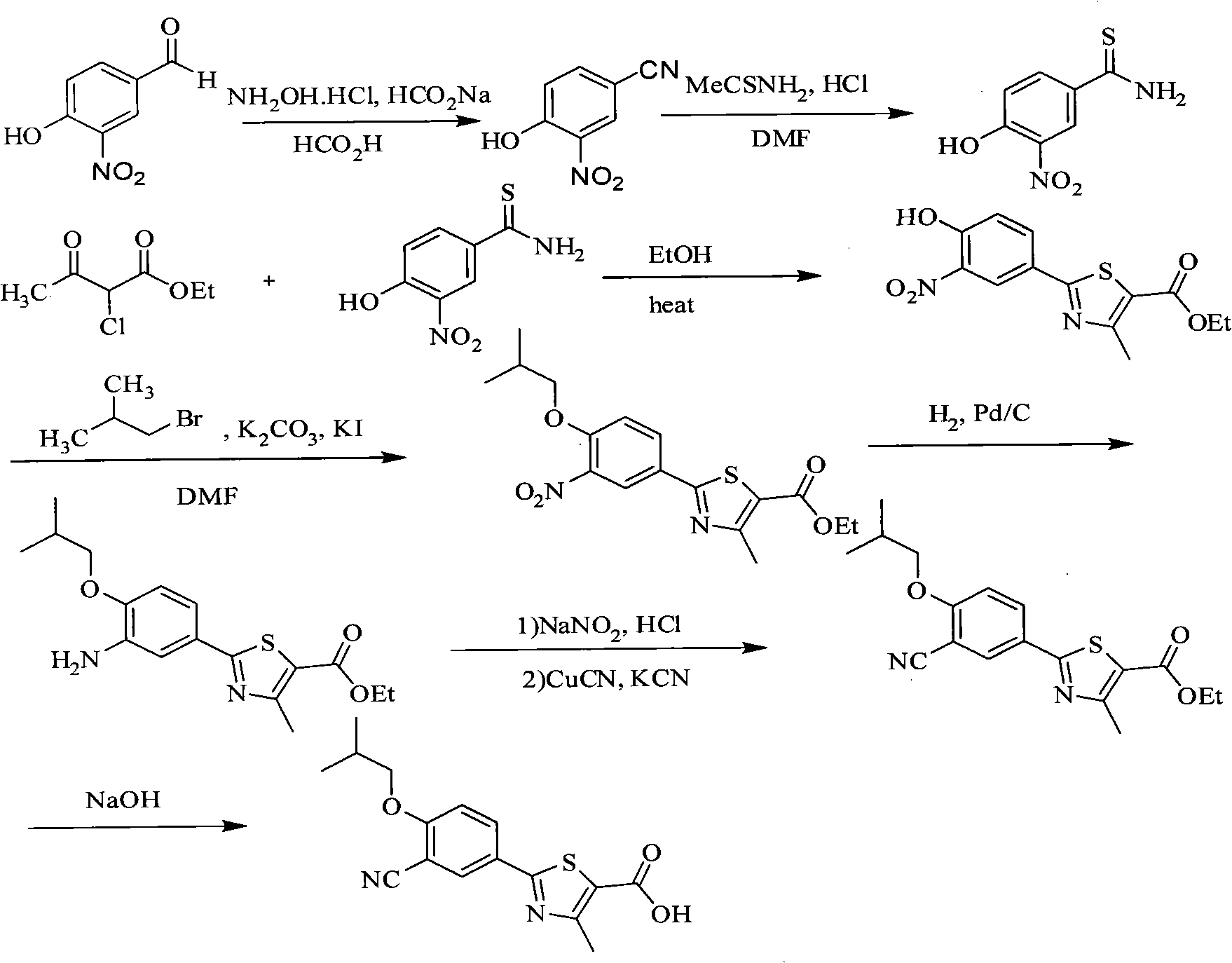
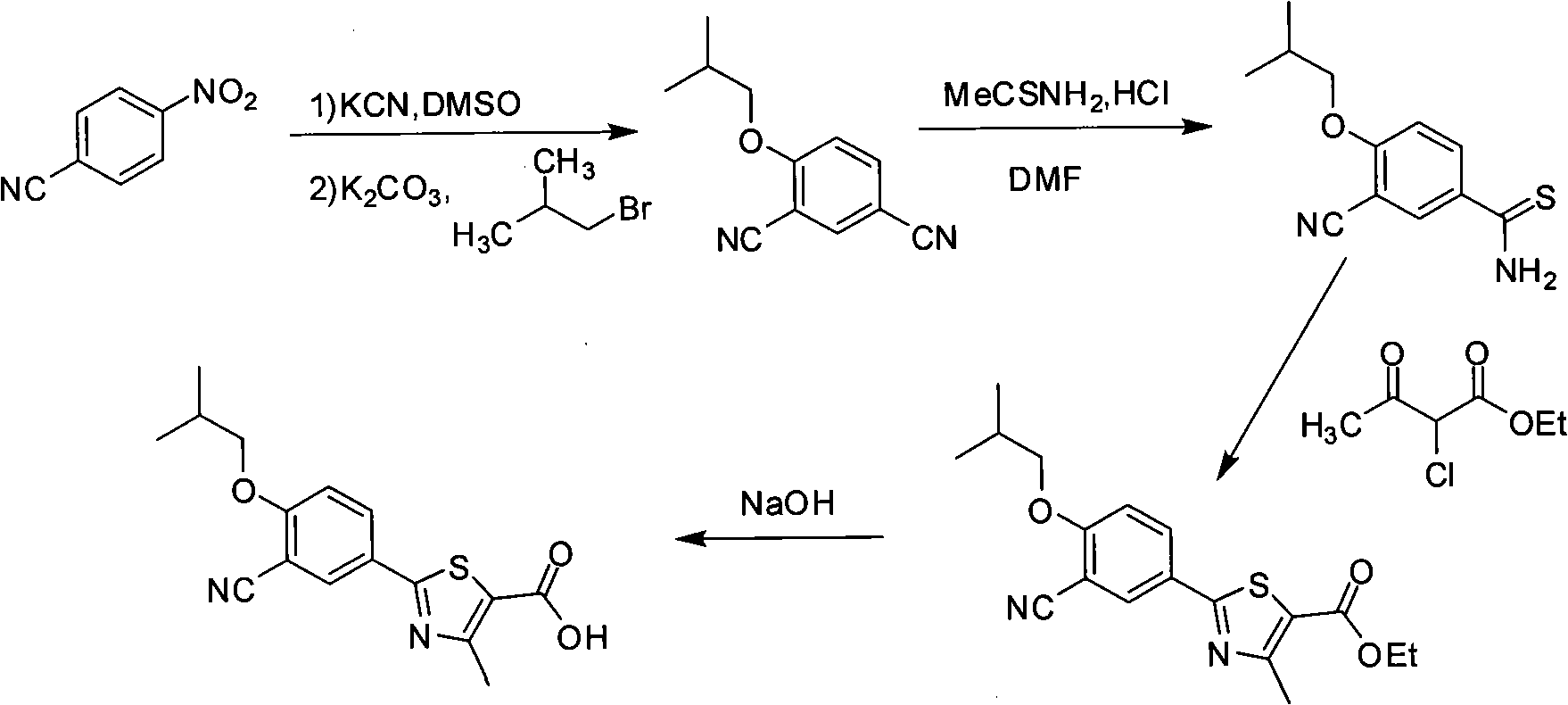
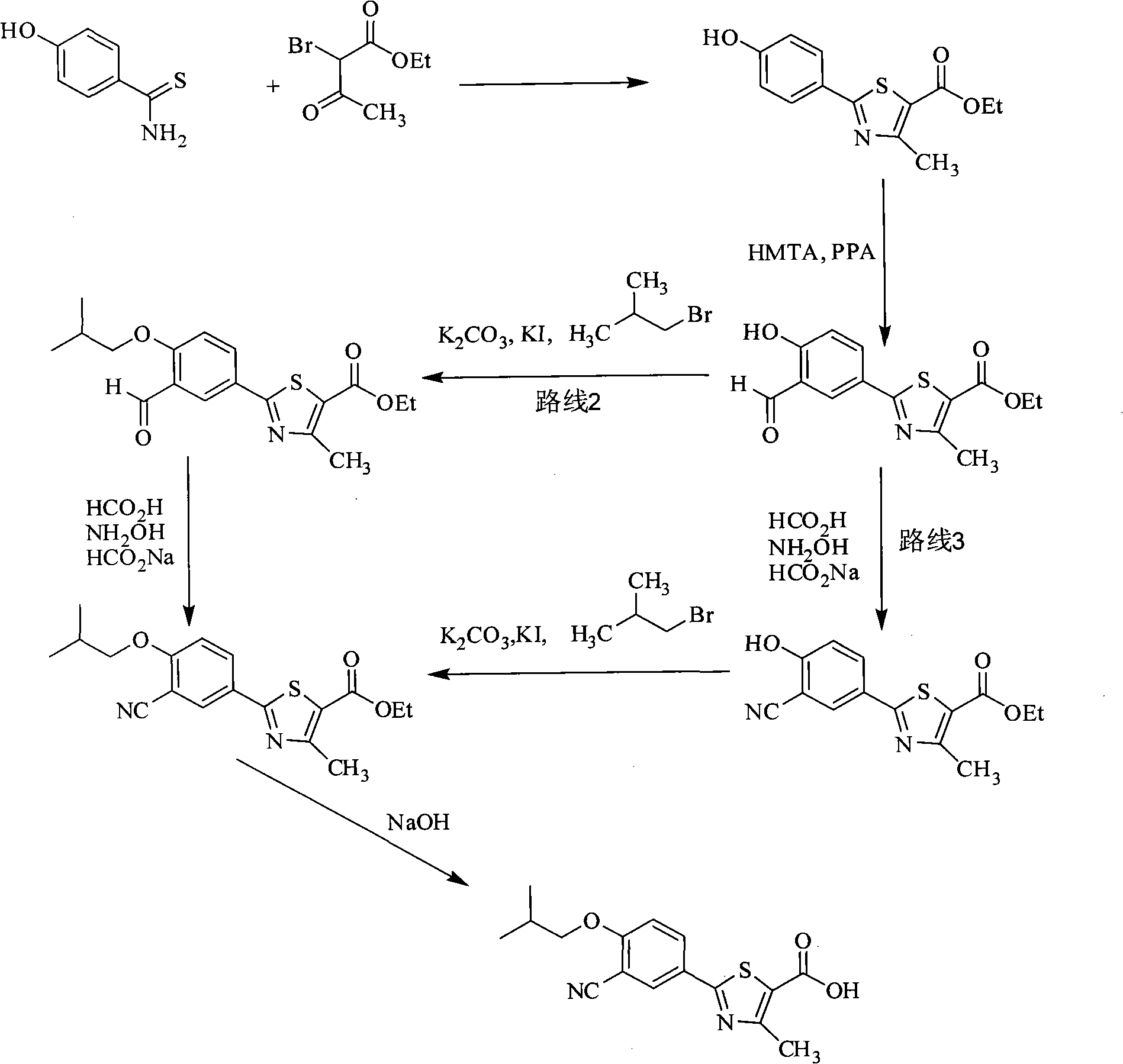
Synthesis method of 2-(3-cyan-4-isobutoxy) phenyl-4-methyl-5-thiazole formic acid
A technology of isobutoxyphenyl thiocarboxamide and isobutoxybenzonitrile is applied in the field of synthesis of 2-phenyl-4-methyl-5-thiazole formic acid, and can solve the problem of high price and increase production Cost and other issues, to achieve the effect of low cost, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Preparation of 3-bromo-4-hydroxybenzonitrile
[0049] Add 10g of 4-hydroxybenzonitrile, 60mL of dichloromethane and 0.5g of iodine into a 250mL three-necked flask, stir at -5°C under temperature control, and slowly add 26.7g of bromine dropwise, after completion of the dropwise reaction, stir at room temperature for 18h . Pour the reaction solution into 110mL of NaHSO with a mass fraction of 16% 3 The solution was stirred for 30 min, filtered with suction, washed with water, and dried to obtain 14.9 g of white solid with a yield of 89.5%. EI-MS: m / z 197.0 [M-H] - . mp: 154-155°C (Document: mp: 155-156°C, J. Org. Chem. 62(13), 4504-4506, 1997).
[0050] (2) Preparation of 3-bromo-4-isobutoxybenzonitrile
[0051] Add 14.9g of 3-bromo-4-hydroxybenzonitrile, 15.0g of triethylamine, and 75mL of acetone into a 250mL round bottom flask, stir, then add 20.6g of bromoisobutane and 0.75g of potassium iodide, at 80°C Reaction 6h. Distillation under reduced pressure gave...
Embodiment 2
[0063] (1) Preparation of 3-bromo-4-hydroxybenzonitrile
[0064] Add 20g of 4-hydroxybenzonitrile, 150mL of dichloromethane and 1g of iodine into a 250mL three-necked flask, stir at 0°C under temperature control, and slowly add 53.4g of bromine dropwise, after the dropwise completion, stir at room temperature for 24h. Pour the reaction solution into 440mL of NaHSO with a mass fraction of 16% 3 solution, stirred for 30 min, filtered with suction, washed with water, and dried to obtain 30.6 g of white solid with a yield of 92.0%.
[0065] (2) Preparation of 3-bromo-4-isobutoxybenzonitrile
[0066] Add 30.6g of 3-bromo-4-hydroxybenzonitrile, 42.3g of anhydrous potassium carbonate, and 150mL of DMF into a 500mL round-bottomed flask, stir, then add 62.4g of bromoisobutane and 1.5g of PEG400, at 60°C Reaction 8h. Suction filtration while hot, wash the filter cake with DMF, and distill the filtrate under reduced pressure to obtain a yellow oil, pour it into water, extract with chl...
Embodiment 3
[0076] (1) Preparation of 3-bromo-4-hydroxybenzonitrile
[0077] Add 60g of 4-hydroxybenzonitrile, 480mL of dichloromethane and 3g of iodine into a 500mL three-necked flask, stir at -5°C under temperature control, and slowly add 160.3g of bromine dropwise. Pour the reaction solution into 660mL of NaHSO with a mass fraction of 16% 3 The solution was stirred for 30 min, filtered with suction, washed with water, and dried to obtain 89.8 g of white solid, with a yield of 90.7%.
[0078] (2) Preparation of 3-bromo-4-isobutoxybenzonitrile
[0079] Add 89.8g of 3-bromo-4-hydroxybenzonitrile, 71.7g of pyridine, and 450mL of ethanol into a 500mL round bottom flask, stir, then add 125.2g of bromoisobutane and 8g of PEG400, and react at 50°C for 8h. Distillation under reduced pressure gave a yellow oil, which was poured into water and extracted with dichloromethane. The combined organic layers were added with anhydrous sodium sulfate and dried. Filter and distill to obtain 118.7 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com