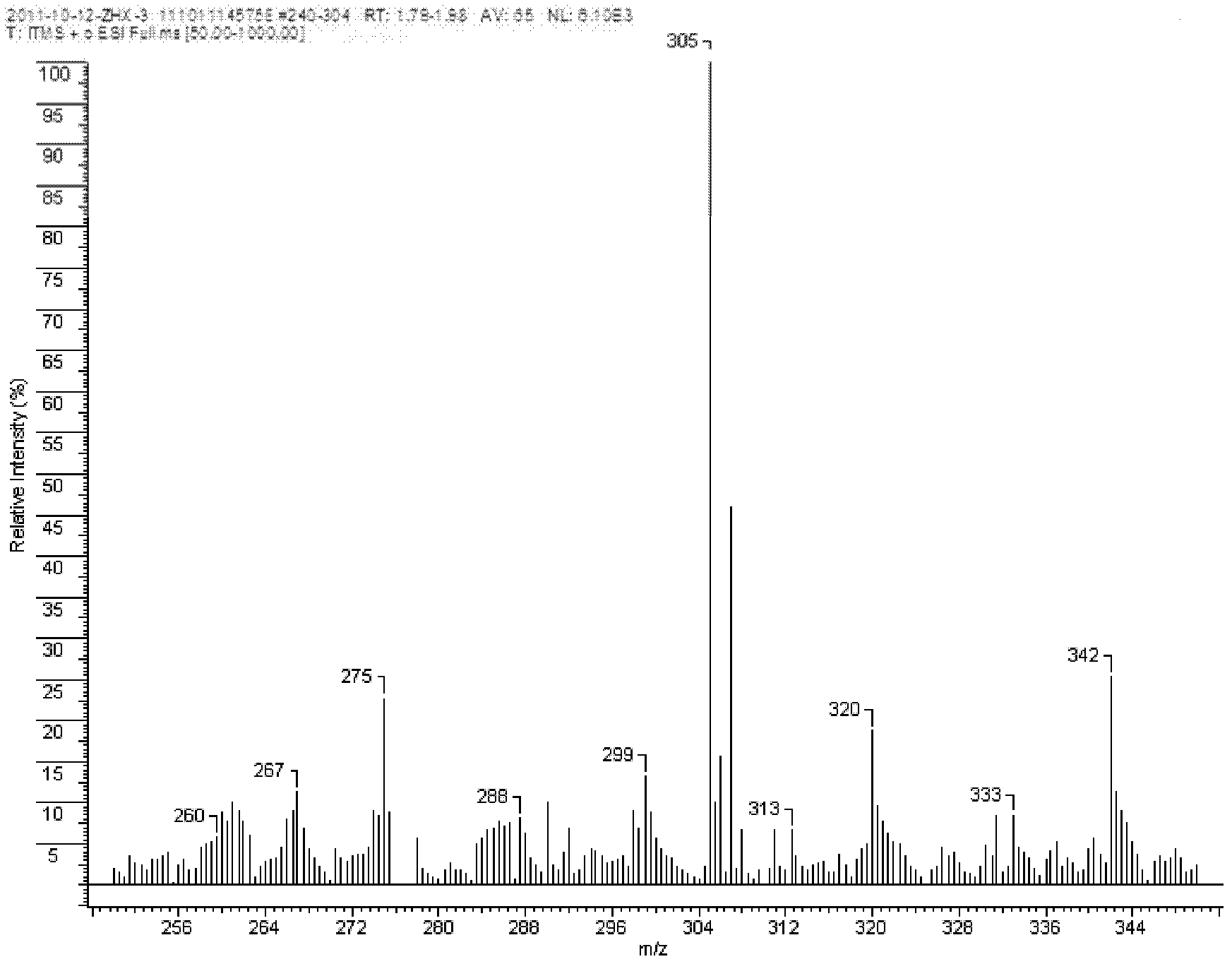
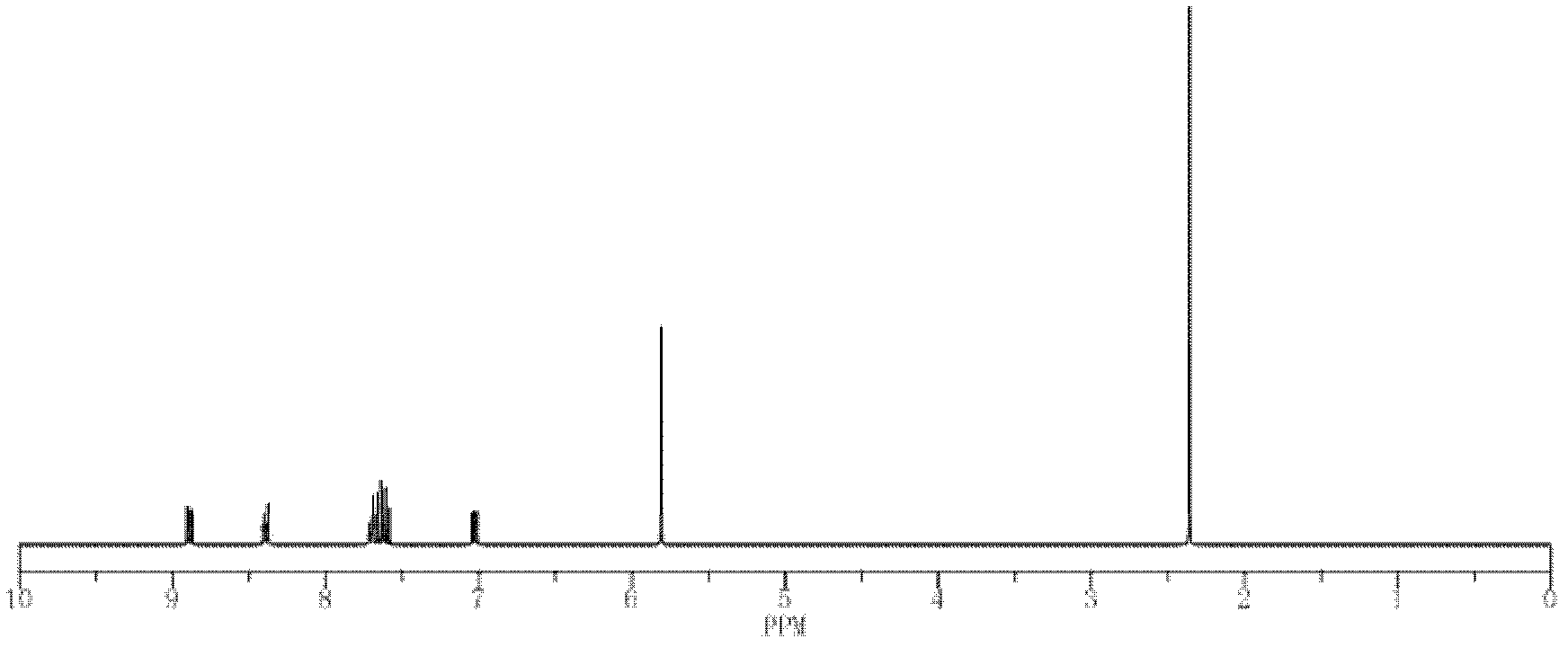
Oxazolyl quinoline organic copper compound, preparation method of the oxazolyl quinoline organic copper compound, oxazolyl quinoline organic copper compound agent, and use of the oxazolyl quinoline organic copper compound in prevention and treatment of crop diseases
A technology of oxazoquinolines and compounds, applied in the field of prevention and treatment of agricultural plant diseases, oxazoquinoline organic copper compounds and their preparation methods, and preparations, can solve the problems of weak effect, low cost, and increased control costs, and achieve Ease of industrial production, mild reaction conditions, and good cost advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve 9.6g of hydroxylamine hydrochloride (industrial product) with a purity of 98% in 100ml of pure water, add 50ml of a 30% mass fraction of sodium hydroxide aqueous solution to neutralize after cooling to 5°C to obtain a mixed solution, and mix the solution dropwise (0.02-0.04ml per drop) was added dropwise to 100ml of ethyl acetoacetate, and the dropwise addition was completed within 15 minutes. The temperature was controlled at 5°C, and the reaction was stirred for 1.5 hours. After the reaction was completed, 30ml of concentrated sulfuric acid with a mass fraction of 98% was added. Stir the reaction at 25°C for 2h, concentrate in vacuo after the reaction to obtain light yellow crystals, heat the light yellow crystals with benzene to reflux for 2h, evaporate the solvent, and finally recrystallize with n-hexane to obtain the compound 3-hydroxy-5-methyliso Oxazole, get the 3-hydroxyl-5-methylisoxazole that 4.6g makes, 7.4g purity is 98% 8-hydroxyquinoline and 240ml ...
Embodiment 2
[0037] The preparation method of copper-3-hydroxyl-5-methylisoxazole-8-hydroxyquinoline is the same as in Example 1.
[0038] Take the following materials in percentage by weight: 20.0% of copper-3-hydroxyl-5-methylisoxazole-8-hydroxyquinoline prepared in this example, 1.0% of octylphenol polyoxyethylene ether, two 4.5% sodium butylnaphthalene sulfonate formaldehyde condensate, 74.5% white carbon black, according to the above ratio, the materials are fully mixed, ground and passed through a 320-mesh sieve to obtain a 20% oxazoquinoline organocopper compound wettable powder.
[0039] The test strain is rice bacterial blight, which is provided by the Pesticide Research Institute of Northwest Agriculture and Forestry University. The prepared 20% oxazoquinoline organocopper compound wettable powder is determined by routine antibacterial tests according to conventional methods. The wettable powder of %hymexazol or carbendazim (the auxiliary materials and their contents are all the ...
Embodiment 3
[0041] Get 5.0g commercially available purity and be 98% hymexazol, 7.6g purity be 98% 8-hydroxyquinoline and 200ml mass fraction be the methanol aqueous solution of 95% one-time drop in 500ml reaction bottle, at the temperature of 85 ℃ Heat and dissolve under low temperature to obtain solution A; add 2.5g of copper sulfate pentahydrate with a purity of 99% and 25ml of distilled water into a 500ml reaction flask, heat, and stir at a temperature of 40°C to fully dissolve it. After complete dissolution, add solution A drop by drop (0.02-0.04ml per drop) to the aqueous solution of copper sulfate under the action of stirring, complete the dropwise addition within 25 minutes, then control the temperature at 65°C, and stir for 1 hour After the reaction, let it stand for precipitation for 4 hours. After the layers are fully separated, remove the upper layer solution, and the dark green precipitate in the lower layer is washed with absolute ethanol (or ethanol) and water in turn, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com