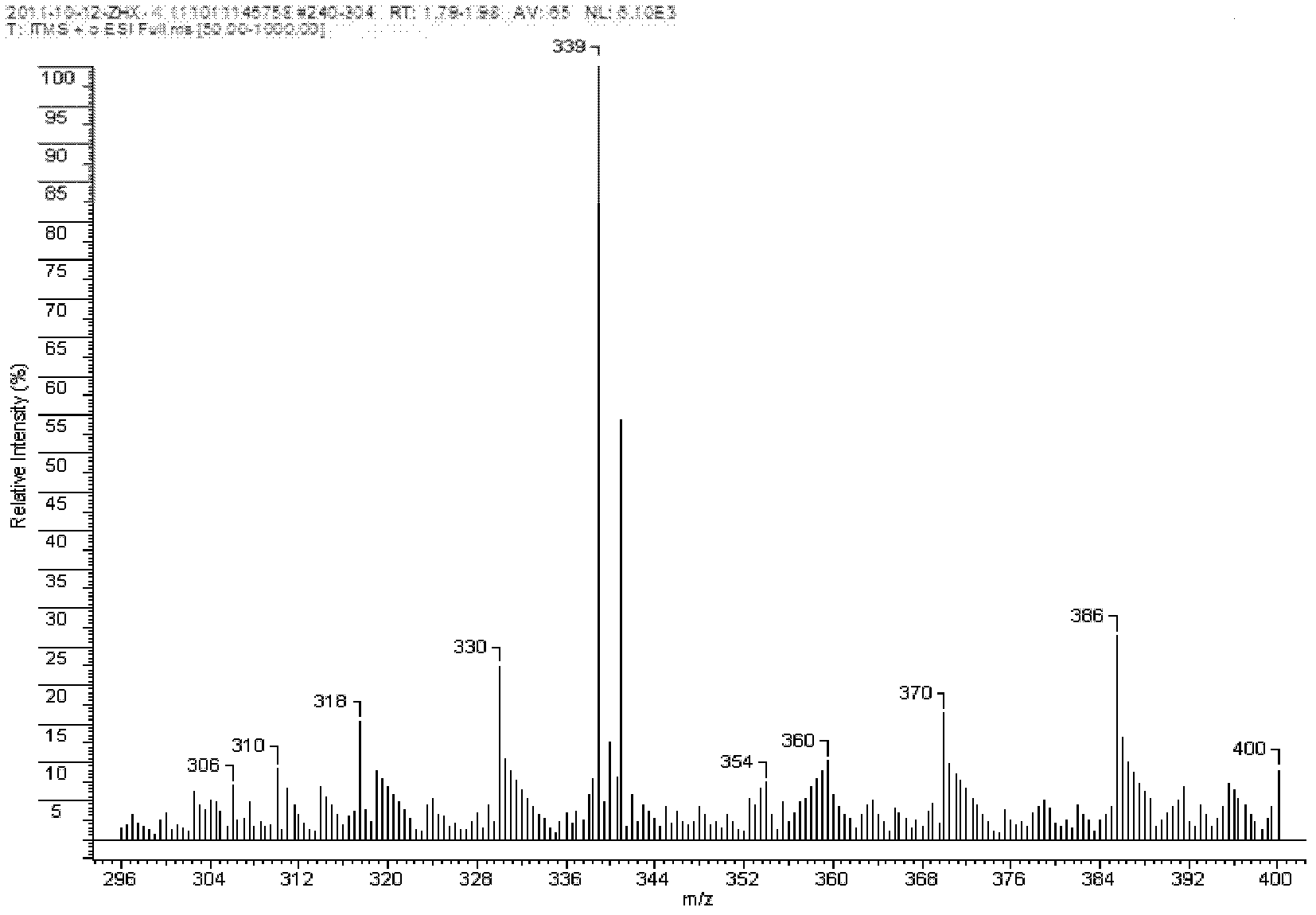
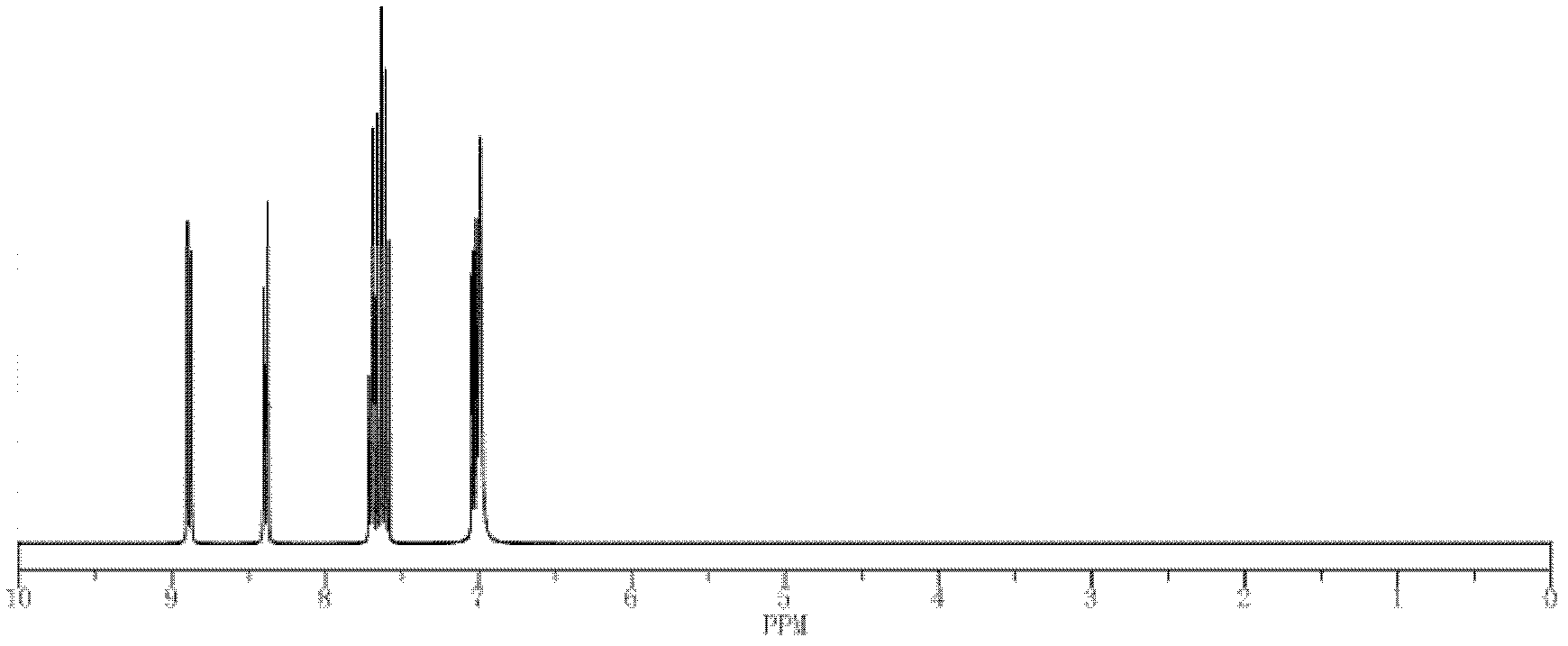
Thiadiazole quinoline organic copper compound, preparation method thereof, preparation thereof, and application thereof in controlling agricultural plant diseases
A thiadiazoquinoline and compound technology are applied in the field of preventing and treating agricultural plant diseases, thiadiazoquinoline organocopper compounds and their preparation fields, and can solve the problems of sensitive plant phytotoxicity, rust tick value-added, crop phytotoxicity and the like , to achieve the effect of easy industrial production, mild reaction conditions and broad sterilization spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]5.6g of 99% 2-amino-5-mercapto-1,3,4-thiadiazole, 6.4g of 98% 8-hydroxyquinoline and 260ml of 75% ethanol solution in one go Put it into a 500ml reaction bottle, heat and dissolve at a temperature of 75°C to obtain solution A, add 2.5g of copper sulfate pentahydrate with a purity of 99% and 25ml of distilled water into a 500ml reaction bottle, heat, and at a temperature of 42°C Stir to fully dissolve, after the copper sulfate is completely dissolved in water, under the action of stirring, add solution A dropwise (0.02-0.04ml per drop) into the aqueous solution of copper sulfate, and the dropwise addition is completed within 27 minutes , then temperature controlled at 70°C, stirred for 1 hour, allowed to stand for precipitation for 4.5 hours after reaction, after fully layered, removed the upper layer solution, and the grayish-brown precipitate in the lower layer was washed with absolute ethanol (or ethanol) and water successively, and then kept at a constant temperature ...
Embodiment 2
[0035] The preparation method of copper-2-amino-5-mercapto-1,3,4-thiadiazole-8-hydroxyquinoline is the same as in Example 1.
[0036] Get the following materials in percentage by weight: Copper-2-amino-5-mercapto-1,3,4-thiadiazole-8-hydroxyquinoline 20.0% prepared in this example, octylphenol polyoxyethylene Base ether 1.0%, dibutyl naphthalene sulfonate sodium formaldehyde condensate 4.5%, white carbon black 74.5%, mix each material fully according to the above ratio, grind and pass through a 320 mesh sieve to get 20% thiadiazoquinolines Organocopper compound wettable powder.
[0037] The tested strain is rice bacterial blight, which is provided by the Pesticide Research Institute of Northwest Agriculture and Forestry University. The prepared 20% thiadiazoquinoline organocopper compound wettable powder is determined by routine antibacterial tests according to conventional methods. 20% carbendazim wettable powder (the excipients and content are the same as the 20% thiadiazoqu...
Embodiment 3
[0039] 5.5g of 99% 2-amino-5-mercapto-1,3,4-thiadiazole, 6.6g of 98% 8-hydroxyquinoline and 240ml of 95% mass fraction of methanol aqueous solution Put it into a 500ml reaction bottle, heat and dissolve at a temperature of 95°C to obtain solution A, add 2.5g of copper sulfate pentahydrate with a purity of 99% and 25ml of distilled water into a 500ml reaction bottle, heat, and at a temperature of 40°C Stir to fully dissolve, after the copper sulfate is completely dissolved in water, add solution A dropwise (0.02-0.04ml per drop) to the aqueous solution of copper sulfate under the action of stirring, and complete the dropwise addition within 25 minutes , then control the temperature at 68°C, stir and react for 1h, let it stand for 4h after the reaction, and remove the upper layer solution after fully layering, and wash the gray-brown precipitate in the lower layer with absolute ethanol (or ethanol) and water successively, and then dry at constant temperature Dry it in an oven at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com