Process for combining glycerol triacetate through acid ion liquid catalyst
A technology of glycerol triacetate and acidic ionic liquid, which is applied in the field of glycerol triacetate synthesis by acidic ionic liquid catalysts, can solve the problems of catalyst stability affecting the mechanical strength of the catalyst, waste water and waste residue discharge, and environmental protection pressure, etc., to eliminate Effect of alkali refining process, elimination of emissions, cleaning of production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
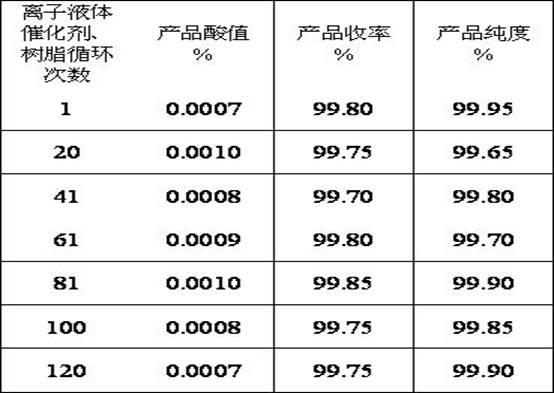
[0022] Weigh 50 g of glycerin (chemically pure), 150 g of acetic acid (chemically pure), 100 g of n-propyl acetate (chemically pure), and Catalyst (BILE-3, alkyl imidazole sulfonate, produced by Enze Biomass Fine Chemical Beijing Key Laboratory) 10g, put into a 500ml glass reactor; the glass reactor is connected to a glass rectifier with 20 theoretical plates Tower, react at 100°C and stirring for 3 hours, control the temperature at the top of the rectification tower between 82-85°C; add 7.5g of acetic anhydride to the reaction kettle after reacting for 3 hours, continue at 100°C and stirring React for 1 hour; after the reaction finishes, open the vacuum pump at the top of the tower to maintain the vacuum of the reaction system at 50KPa, steam the reaction solvent n-propyl acetate and excess acetic acid, and use the mixture of solvent n-propyl acetate and acetic acid as the next batch of raw materials. The residue in the kettle is a mixture of catalyst ionic liquid and product...
Embodiment 2
[0024] Weigh 100 g of glycerin (chemically pure), 350 g of acetic acid (chemically pure), 150 g of n-propyl acetate (chemically pure), and Catalyst (BILE-3, produced by Enze Biomass Fine Chemicals Beijing Key Laboratory) 30g was added to a 1000ml glass reactor; a glass rectification tower with a theoretical plate number of 20 was connected to the glass reactor, and stirred at 120°C Under the condition of reaction for 3 hours, the temperature of the top of the rectification tower is controlled between 82-85°C; after 3 hours of reaction, add 20g of acetic anhydride to the reaction kettle, and continue to react for 1 hour at 120°C under stirring conditions; after the reaction, Turn on the vacuum pump at the top of the tower, maintain the vacuum degree of the reaction system at 20KPa, distill off the reaction solvent n-propyl acetate and excess acetic acid, the mixture of solvent n-propyl acetate and acetic acid is used as the next batch of raw materials, and the remainder in the r...
Embodiment 3
[0026]Weigh 100 g of glycerin (chemically pure), 400 g of acetic acid (chemically pure), 200 g of n-propyl acetate (chemically pure), and Catalyst (BILE-3, produced by Enze Biomass Fine Chemicals Beijing Key Laboratory) 40g was added to a 1000ml glass reactor; a glass rectification tower with a theoretical plate number of 20 was connected to the glass reactor, and stirred at 140°C Under the condition of reaction for 3 hours, control the temperature of the top of the rectification tower between 82-85°C; after reacting for 3 hours, add 30g of acetic anhydride to the reactor, and continue to react for 1 hour at 140°C under stirring conditions; after the reaction, Turn on the vacuum pump at the top of the tower, maintain the vacuum degree of the reaction system at 30KPa, distill off the reaction solvent n-propyl acetate and excess acetic acid, the mixture of solvent n-propyl acetate and acetic acid is used as the next batch of raw materials, and the remainder in the reaction kettle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com