Bridged triphenylamine-based polymer solar battery
A technology of solar cells and polymers, which is applied in the field of polymer solar cells, can solve problems such as absorption spectrum mismatch, efficiency not exceeding 5%, low short-circuit current density, etc., achieve low preparation cost, improve photoelectric conversion efficiency, Effect of Improving Carrier Mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
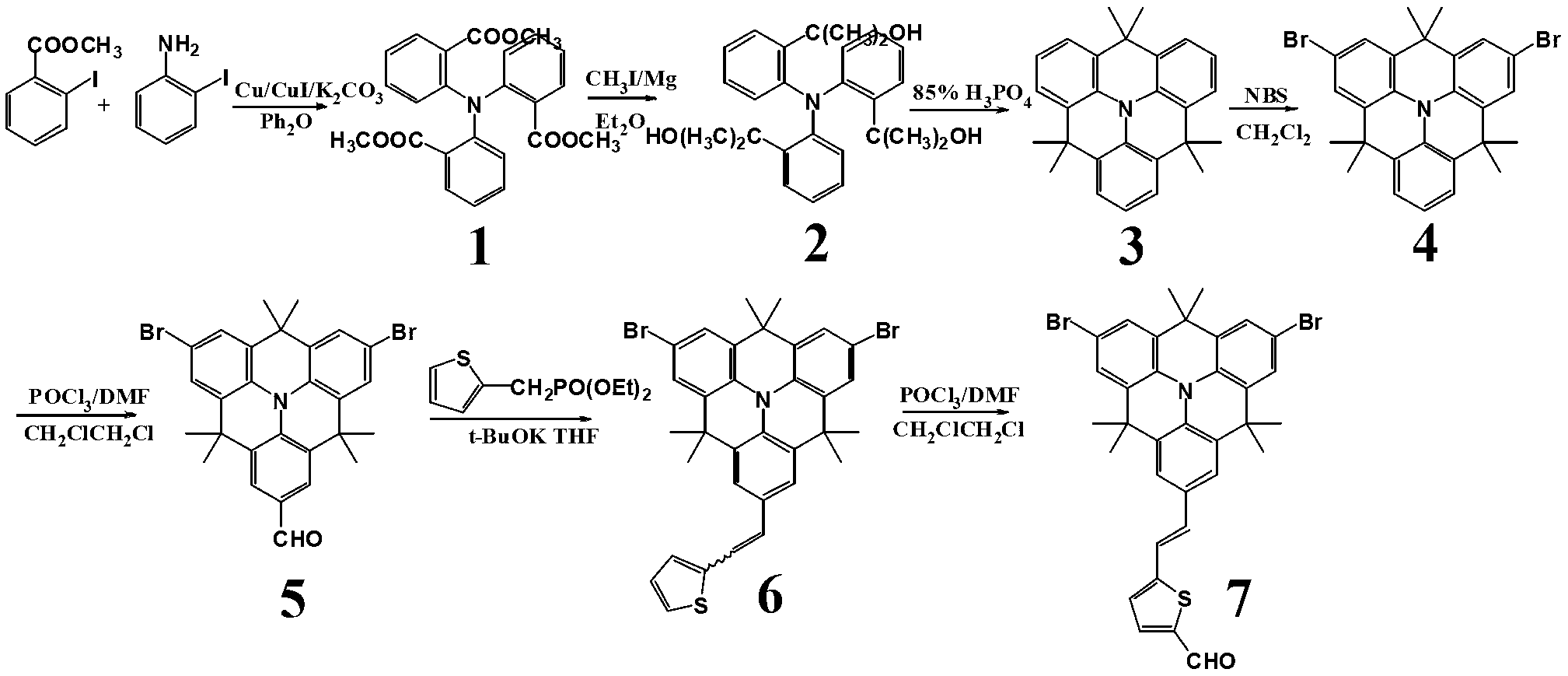
[0028] (1) Synthesis of small molecules
[0029] Synthesis of compound 1
[0030] Add 15.0mL of methyl o-iodobenzoate, 4.50mL of methyl anthranilate, 11.0g of potassium carbonate, 0.45g of activated copper powder, and 0.65g of cuprous iodide into 40mL of diphenyl ether as a solvent. React in an oil bath at 190°C for 48 hours. The solvent diphenyl ether was distilled off under reduced pressure, and then separated and purified by column chromatography. The eluent was dichloromethane:ethyl acetate=15:1. A pale yellow solid was obtained with a yield of 9.82 g and a yield of 67.2%. 1 H-NMR (300MHz, CDCl 3 , δ, ppm) 7.59 (m, 3H), 7.34 (m, 3H), 7.08 (m, 6H), 3.37 (s, 9H).
[0031] Synthesis of compound 2
[0032] Under an argon atmosphere, add 3.00g of magnesium chips to 50mL of anhydrous ether, drop 17.0g of methyl iodide at 0°C, add a small grain of iodine to initiate, and reflux for 1 hour. 4.19 g of compound 1 was dissolved in 50 mL of toluene, slowly dropped into the above...
Embodiment 2
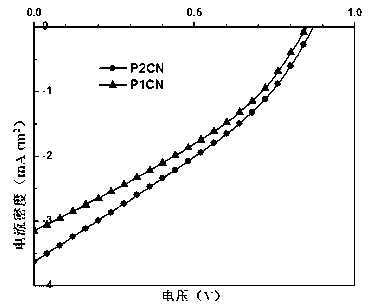
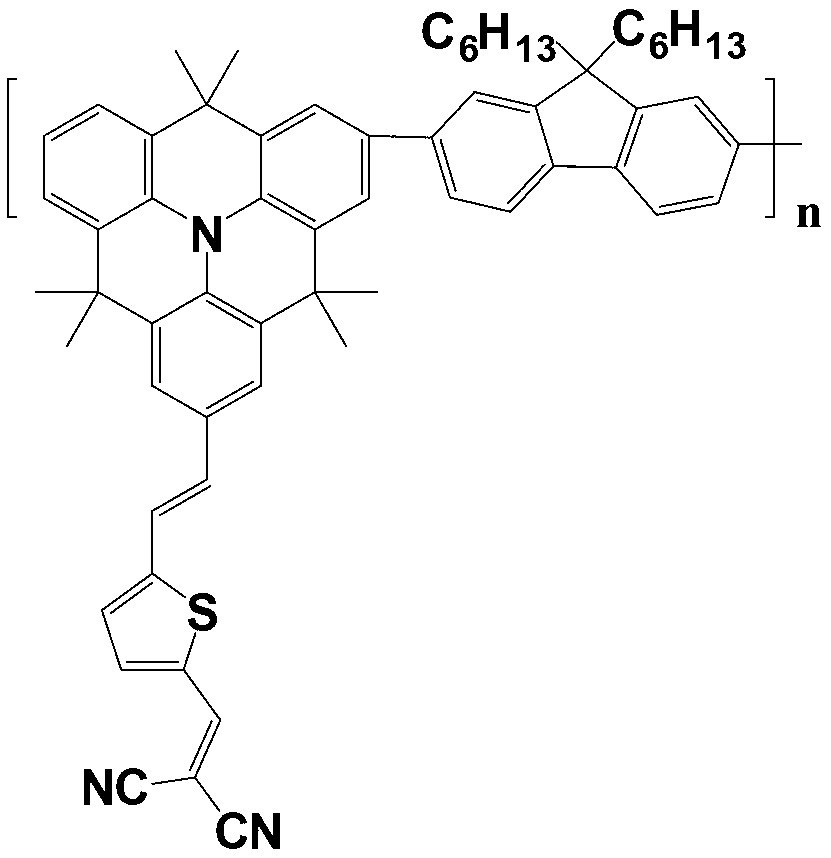
[0053]Wash the ITO glass with deionized water / acetone and then ultrasonically shake it, spin-coat the aqueous solution of PEDOT and PSS on the surface of the ITO glass, spin and centrifuge at 500r / min for 2 minutes, and dry to obtain a composite film of PEDOT:PSS; P1CN and PCBM The mixed o-dichlorobenzene solution with a weight ratio of 1:1 was heated to 110°C and then coated, and then dried in vacuum after rotating and centrifuging at 500r / min for 2 minutes to obtain an active layer composite film; spin-coat PFN on the active layer as a cathode modification layer; finally A layer of 100nm-thick aluminum is vacuum-evaporated as a cathode to obtain a complete polymer solar cell device (P2CN as a donor polymer prepares a polymer solar cell with the same steps as P1CN). The performance comparison of solar cell devices prepared by P1CN and P2CN respectively as donor polymers is shown in Table 1:
[0054] Table 1. Performance of solar cell devices prepared by P1CN and P2CN respecti...
Embodiment 3
[0058] Wash the ITO glass with deionized water / acetone and then ultrasonically shake it, spin-coat the aqueous solution of PEDOT and PSS on the surface of the ITO glass, spin and centrifuge at 500r / min for 2 minutes, and dry to obtain a composite film of PEDOT:PSS; P1CN and PCBM Heat the mixed o-dichlorobenzene solution with a weight ratio of 1:1 to 110°C and apply it, spin and centrifuge at 500r / min for 2 minutes and then dry in vacuum to obtain an active layer composite film, anneal at 100°C for 10 minutes; spin-coat PFN on the active layer As a cathode modification layer; finally, a layer of aluminum with a thickness of 100nm is vacuum evaporated as a cathode to obtain a complete polymer solar cell device (P2CN as a donor polymer prepares a polymer solar cell with the same steps as P1CN). The performance comparison of solar cell devices prepared by P1CN and P2CN as donor polymers is shown in Table 2:
[0059] Table 2. Performance of solar cell devices prepared with P1CN and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com