Bone-targeted RNA interference compound and synthetic method thereof
A technology of RNA interference and complexes, applied in the field of biomedicine, can solve the problems of complex operation, difficulty in drug delivery to organs, and transfection of cells, and achieve easy separation and purification, avoid side effects, and expand the scope of basic research Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of siRNA-(STR-R8)-(D-Asp8) complex
[0036] Synthetic steps
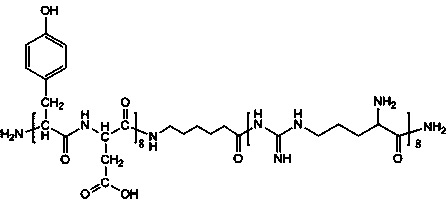
[0037] a. Mix D-Asp8 containing acetylcysteine residues with STR-R8 liposomes (30 μmol / ml) at a molar ratio of 3:1 and incubate at room temperature for 2 hours to obtain (STR-R8)-( D-Asp8) complex, molecular formula see figure 1 .
[0038] b. Using a Sepharose column, remove non-copolymerized D-Asp8 by size exclusion chromatography.
[0039] c. Take 0.5 ml of copolymerized (STR-R8)-(D-Asp8) liposome suspension and 0.5 ml of mannitol aqueous solution (mannitol to water molar ratio = 5:1) and freeze-dry for 48 hours.
[0040] d. Dissolve the lyophilized liposome complex (STR-R8)-(D-Asp8) with HEPES (4-hydroxyethylpiperazineethanesulfonic acid, pH 4.0, concentration 10mM) to a final concentration of 0.17 mg / mL .
[0041] e. siRNA encapsulation: Shake and mix 0.1 mg / mL siRNA solution (solvent is HEPES (pH 4.0, concentration 10mM)) and (STR-R8)-(D-Asp8) solution, incubate at room temperature for 2...
Embodiment 2
[0044] Example 2 Validation of (STR-R8)-(D-Asp8) Tissue Targeting
[0045] Experimental procedure
[0046] a. Synthesis of (STR-R8)-(D-Asp8) complex as above and modification with rhodamine
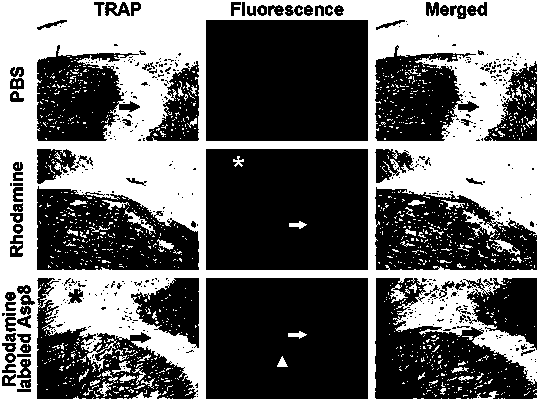
[0047] b. Tail vein injection of targeted gene transfection complex, simple rhodamine or PBS at equal doses of rhodamine, after 30min, 1h, 2h, 4h and 24h, use small animal in vivo imaging system to observe and measure the fluorescence intensity of different tissues and organs and conduct quantitative analysis .
[0048] c. 24 hours after administration as described in b, the femoral tissue was taken for fixation, embedded in hard tissue, sectioned, and the fluorescence distribution and intensity in the femur were observed with a laser confocal microscope.
[0049] d. 24 hours after administration as described in b, the femoral tissue was fixed, dehydrated, frozen and hard tissue sectioned, and TRAP stained, and the fluorescence distribution on the surface of the bone trabecula and t...
Embodiment 3
[0056] Example 3 Application of Targeted RNA Interference against Osteoclast Sema4d Gene
[0057] Experimental procedure
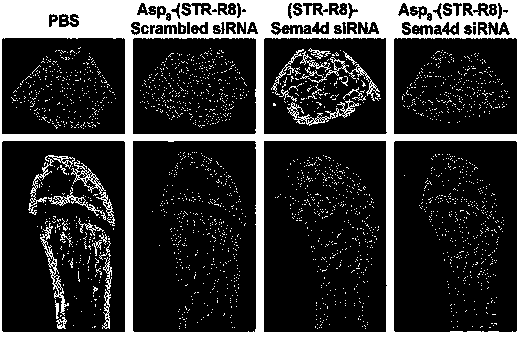
[0058] a. 8-week-old Kunming mice were given treatment measures, the intensity of intervention was once a week, and the intervention period was 4 weeks. Both prevention and treatment experiments were grouped as follows: ①PBS group (blank control), ②Scrambled siRNA-(STR-R8)-(D-Asp8) group (siRNA negative control), ③Sema4d siRNA-(STR-R8) group (non-targeting Negative control), ④OVX+Sema4d si RNA-(STR-R8)-(D-Asp8) group (experimental group). Calcein and alizarin red were injected subcutaneously three days after the intervention and four days before the sampling.
[0059] b. Four weeks after the intervention, the bilateral femurs were fixed with 4% PFA and microCT was performed to analyze the bone mass, bone density and bone microstructure of the distal end of the femur and vertebral cancellous bone.
[0060] c. The other half of the femur after microCT sc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com