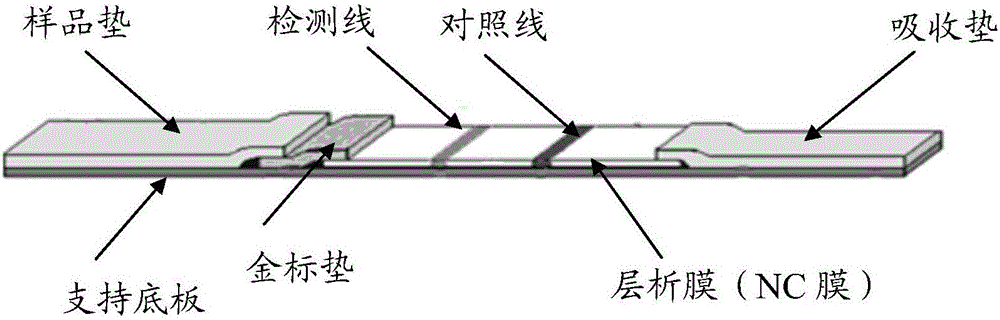
Immuno-gold labeling card for general detection of fluoroquinolones and preparation method thereof
A technology of fluoroquinolones and gold standard cards, applied in the biological field, can solve problems such as poor versatility, false positives, and false negatives of detection accuracy, and achieve the effects of sensitivity and versatility, high versatility, and strong versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
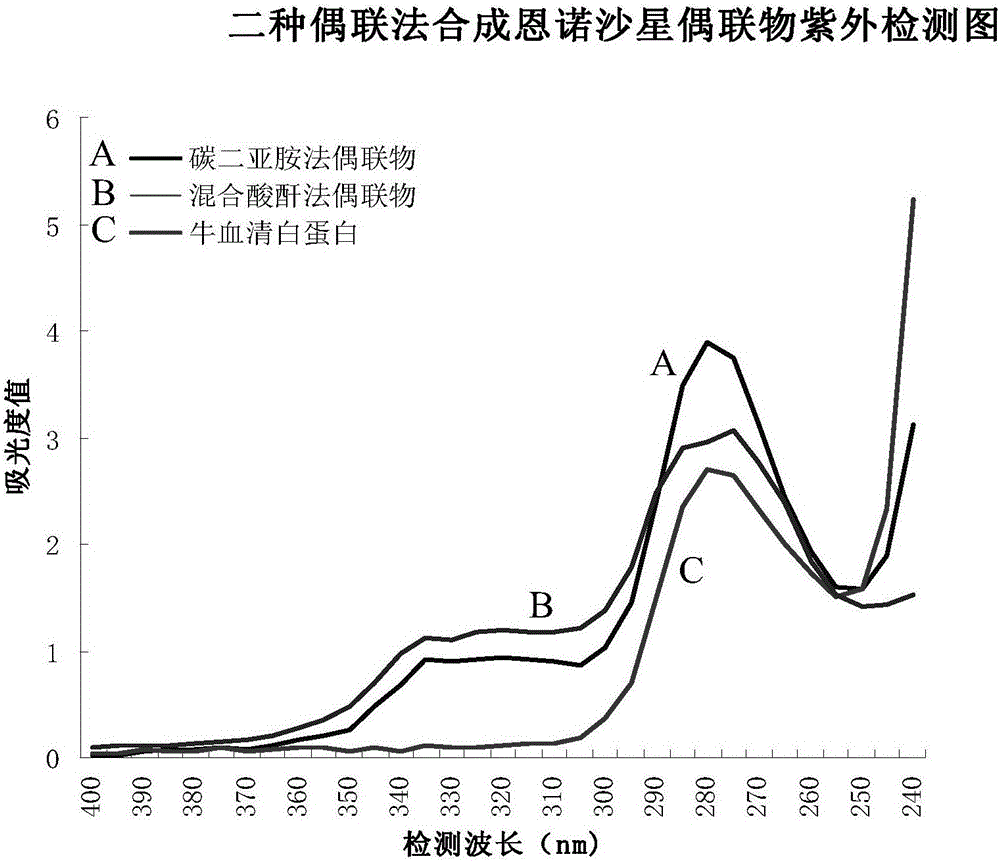
[0050] In the preparation of the immunogold standard card in the present invention, the enrofloxacin standard sample is preferably coupled with high-purity cationized bovine serum albumin (cBSA) by carbodiimide coupling method or mixed anhydride method. The conjugate is prepared as a test line (T line) for the coated antigen. The two methods are as follows:
[0051] 1. Carbodiimide coupling method
[0052] First, mix and dissolve ethylenediamine and bovine serum albumin in 0.01M PBS (pH7.0) solution, under the action of the chemical coupling agent - dicycloethylcarbodiimide, block -COOH in the bovine serum albumin molecule Residues, excess ethylenediamine and dicycloethylcarbodiimide were removed by dialysis, and cationized bovine serum albumin was obtained as a protein carrier.
[0053] Dissolve enrofloxacin (EF) standard sample in N,N-dimethylformamide, add N-hydroxysuccinimide and dicyclohexylcarbodiimide to react overnight at room temperature to form an activated ester; ...
Embodiment 1
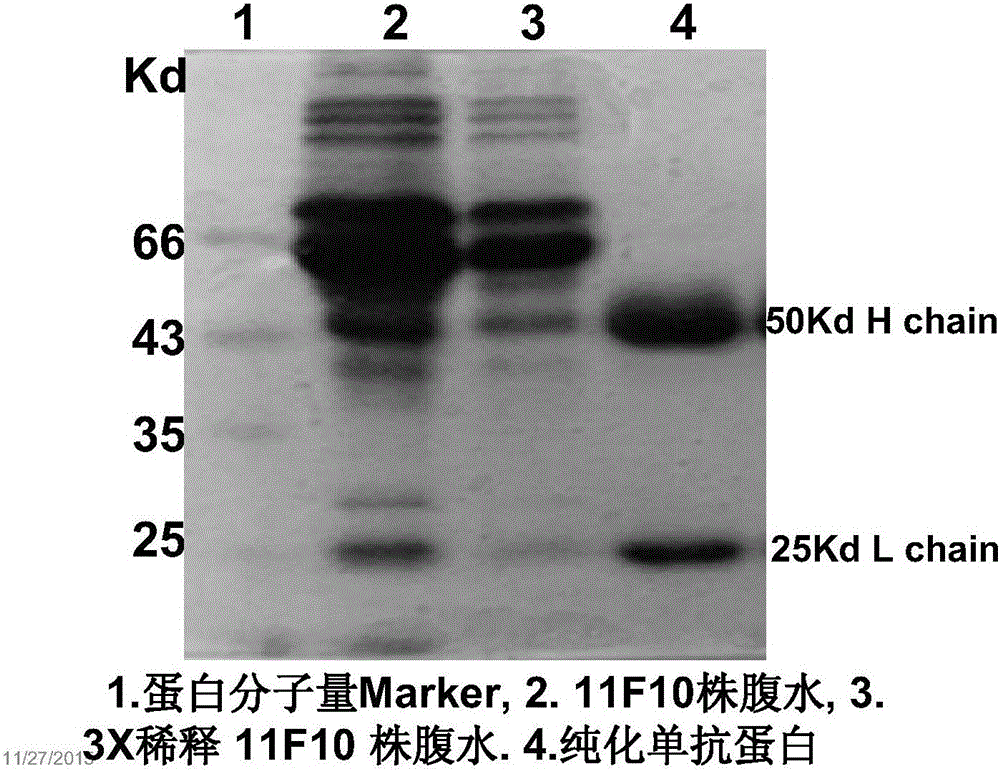
[0059] Embodiment 1: Preparation of the monoclonal antibody protein of the present invention
[0060] 1. Recovery and proliferation of hybridoma cell line -11F10
[0061] Take the cryopreservation tube of the hybridoma cell line-11F10 from the liquid nitrogen tank, melt it rapidly in a water bath at 37°C, centrifuge at 600r / min for 5min, discard the supernatant, add fresh 15% FBS / RPMI-1640 culture medium to suspend the cells, each After adding the above-mentioned culture solution to 5ml, plant it in a 50ml cell culture bottle and culture it in a carbon dioxide incubator. After the cells grow to a density of 30%, change the medium halfway, and when the cells grow to about 60% (in the logarithmic growth phase), pass the culture. Passage at 1:3-4 every 2-3 days.
[0062] 2. In vivo induction of monoclonal antibody protein and ascites acquisition
[0063] 7-10 days before planting hybridoma cells in vivo, 12 male BALB / c mice aged 8-10 weeks were injected intraperitoneally with 0...
Embodiment 2
[0067] Embodiment 2: Purification of the monoclonal antibody protein of the present invention
[0068] The ascites was pre-cooled in an ice-water bath and diluted 4 times with 0.01M PBS (pH 8.0) as a preparation solution for later use. Take 1ml of Protein G Resin for every 50mg of total protein in ascites, fill the gel in a small column for chromatography (10ml), and equilibrate the column with pre-cooled 0.01M PBS (pH 8.0) 50 times the volume of the column bed At room temperature, the preparation solution was filtered through the Protein G Resin column five times (natural flow rate), so that the monoclonal antibody protein in ascitic fluid could fully bind to protein G specifically, and then pre-cooled 0.01M PBS ( pH 8.0) solution to fully wash the chromatography column (natural flow rate) to remove the impurity proteins adhering to the chromatography column.
[0069] Add 100μl 1M Tris-Cl (pH 9.6) to each 1.5ml EP tube in advance, add ice water to pre-cool 0.1M Glycine-HCl (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com