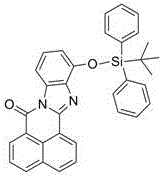
Preparation method and application of benzimidazobenzisoquinolinone silyl ether
A technology of benziisoquinolinone and benzimidazole, which is applied to the application of detecting fluoride ions, the field of preparation of reactive fluoride ion molecular probes, and can solve problems such as blood pressure drop, mitochondrial dysfunction, and immune system metabolic disorders. , to achieve the effect of easy control of conditions, simple preparation process and good linear relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
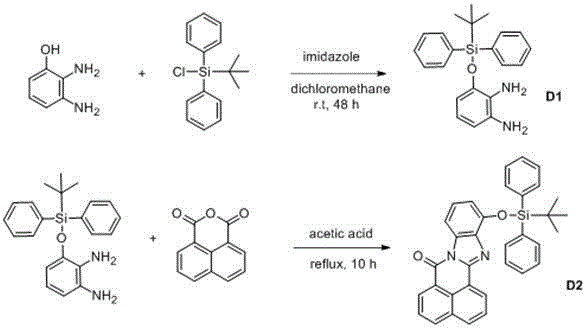
[0022] Example 1: Synthesis of 3-(tert-butyldiphenyl)silyl-1,2-phenylenediamine (intermediate D1)
[0023] Weigh 0.46 g of 2,3-diaminophenol and 0.51 g of imidazole and dissolve them in 40 mL of dry dichloromethane solvent, then add 0.96 mL of tert-butyldiphenylchlorosilane to the solution, and react at room temperature for 48 hours. The reaction solution was concentrated and separated by column chromatography to obtain a light yellow oily liquid with a yield of 73%. NMR mass spectrometry is characterized as follows: 1 H NMR (400 MHz, CDCl 3 ) δ 7.85 (d, J = 6.5 Hz, 4H), 7.47 (dt, J = 14.0, 6.9 Hz, 6H), 6.44 (m, 2H), 6.10 (dd, J = 5.8, 3.6 Hz, 1H), 3.64 (s , 4H), 1.23 (s, 9H).
[0024] Synthesis of Molecular Probe D2
[0025] Weigh intermediate D1 (0.79g), 1,8-naphthalene dicarboxylic anhydride (0.39g) and 10mL glacial acetic acid into a 50mL round bottom flask, and reflux for 10h under nitrogen protection. After the reaction was complete, it was cooled to room tempera...
Embodiment 2
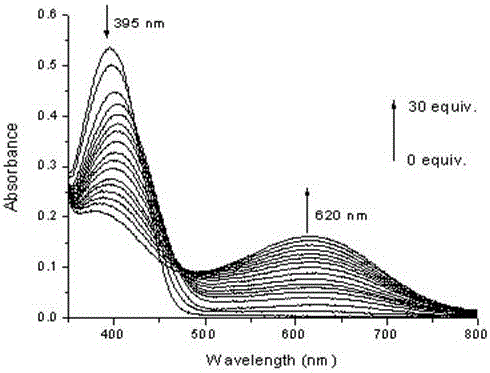
[0027] Probe molecule D2 (1×10 -5 M) in DMSO solution, the measured maximum absorption wavelength is 395nm, and the color is yellow. When F is added dropwise to the solution - (0-30equiv), the system produced a new ultraviolet absorption peak at a wavelength of 620nm, and the maximum absorption wavelength was red-shifted (225 nm). And with F - As the concentration increases, the absorption intensity increases all the time, until the fluoride ion concentration is 30 equiv of the probe concentration, the absorption intensity no longer increases (such as image 3 ), the color of the solution turns blue. Obviously, such a change in the maximum absorption wavelength is due to the breakage of the oxysilyl ether bond (R-O-Si) in the molecular probe and the transformation into an oxyanion (R-O-Si - );
Embodiment 3
[0029] Configuration 1×10 -5 M molecular probe solution, and then 3 equiv of different anions F were added to the solution - , Cl - ,Br - , I - , HSO 4 - , AcO - , H2 PO 4 - , ClO 4 - , to measure the ultraviolet absorption spectrum of the probe molecule in the presence of anions;
[0030] With reference to the ultraviolet absorption spectrum titration experiment of fluoride ion on the probe molecule, the present invention judges the selectivity of the probe to fluoride ion through the competition anion experiment, and adds 3 equiv of different anions F to the solution respectively. - , Cl - , Br - , I - , HSO 4 - ,AcO - , H 2 PO 4 - , ClO 4 - After that, the UV absorption spectrum did not change significantly. Under the same conditions, adding F - After (3equiv), the maximum absorption wavelength red-shifted by 225 nm, and the monitoring of the ultraviolet absorption intensity showed that the present invention is very suitable as a fluoride ion fluoresc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com