Synthesis and application of fluorescent reagent for selectively detecting cysteine based on aggregation-induced emission principle
A cysteine and fluorescent reagent technology, applied in the fields of luminescent materials, fluorescence/phosphorescence, nitro compound preparation, etc., can solve the problems of low detection signal-to-noise ratio, fluorescence quenching, limiting the practical application of Cys fluorescent probes, etc. To achieve the effect of simple synthesis steps, high selectivity, and overcoming the easy bleaching of dye molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Target compound TPENNO 2 Synthesis
[0025]
[0026] (1) Synthesis of Compound 1: Add benzophenone (1.8g, 10mmol) and zinc powder (5.2g, 80mmol) to tetrahydrofuran, cool to -20°C for half an hour while stirring, and then slowly drop in tetrachloride Titanium (4.4mL, 40mmol), continue to stir at -20°C for half an hour, then slowly rise to room temperature, then heat to reflux overnight, cool, filter, concentrate and separate and purify by column chromatography to obtain a compound with aggregation-induced luminescent properties 1. The yield is 72%.
[0027] (2) Synthesis of Compound 2: Compound 1 (2.64g, 8mmol), acetic acid (1.9mL, 32mmol) were added to dichloromethane, and after cooling to -20°C, concentrated nitric acid (65%, 1.6mL, 24mmol ) was added to the reaction liquid, and after stirring at -20°C for 15 min, cold water was added for liquid extraction, the organic phase was washed three times with water, dried and concentrated, and the residue was recrystalli...
Embodiment 2
[0031] Cys selective recognition detection
[0032] (1) Determination of Cys response time: take 50 μL compound TPENNO 2 DMSO solution (1mM) in 2mL PBS buffer solution, adding 200μM Cys, the mixture was incubated at 37°C for different times, and the fluorescence intensity of the mixture solution was measured as a function of the incubation time. The results showed that after 30min, the fluorescence intensity basically reached saturation.
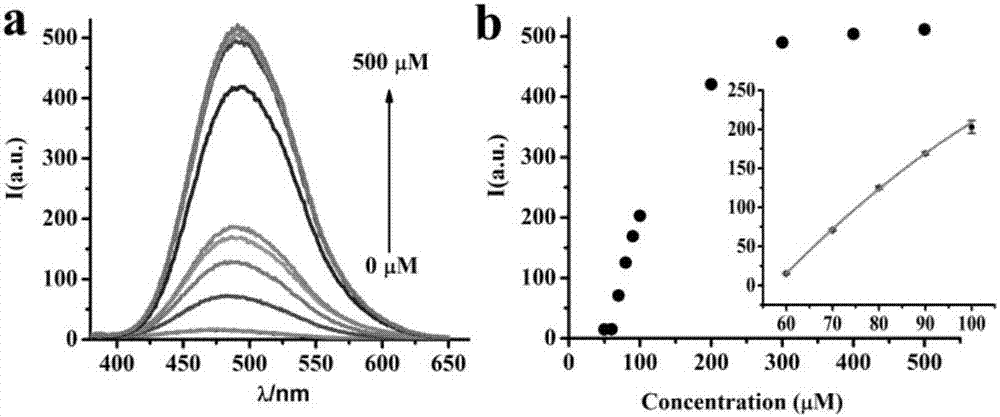
[0033] (2) Cys fluorescence titration test: take 50 μL compound TPENNO 2 DMSO solution (1mM) in 2mL PBS buffer solution, and then add different amounts of Cys in PBS solution, after the mixed solution was incubated at 37°C for 30min, the fluorescence emission spectrum of the solution after adding different Cys was measured (E x =341nm), the fluorescence intensity of the mixed solution showed a good linear relationship in the concentration range of 60 to 100μM Cys (such as figure 1 shown).
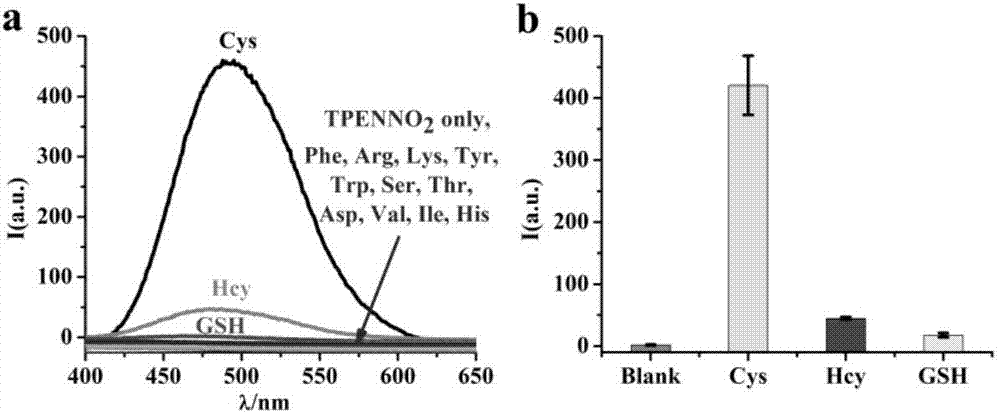
[0034] (3) Compound TPENNO 2 Selectivity test ...
Embodiment 3
[0036] Fluorescence imaging of sulfhydryl compounds in human breast cancer (HeLa) cells: HeLa cells were inoculated in DEME medium containing 10% fetal bovine serum after resuscitation, at 37°C, 5% CO 2 , 100% saturated humidity incubator. Then cultured on 18mm coverslip for 24h, ready to use.
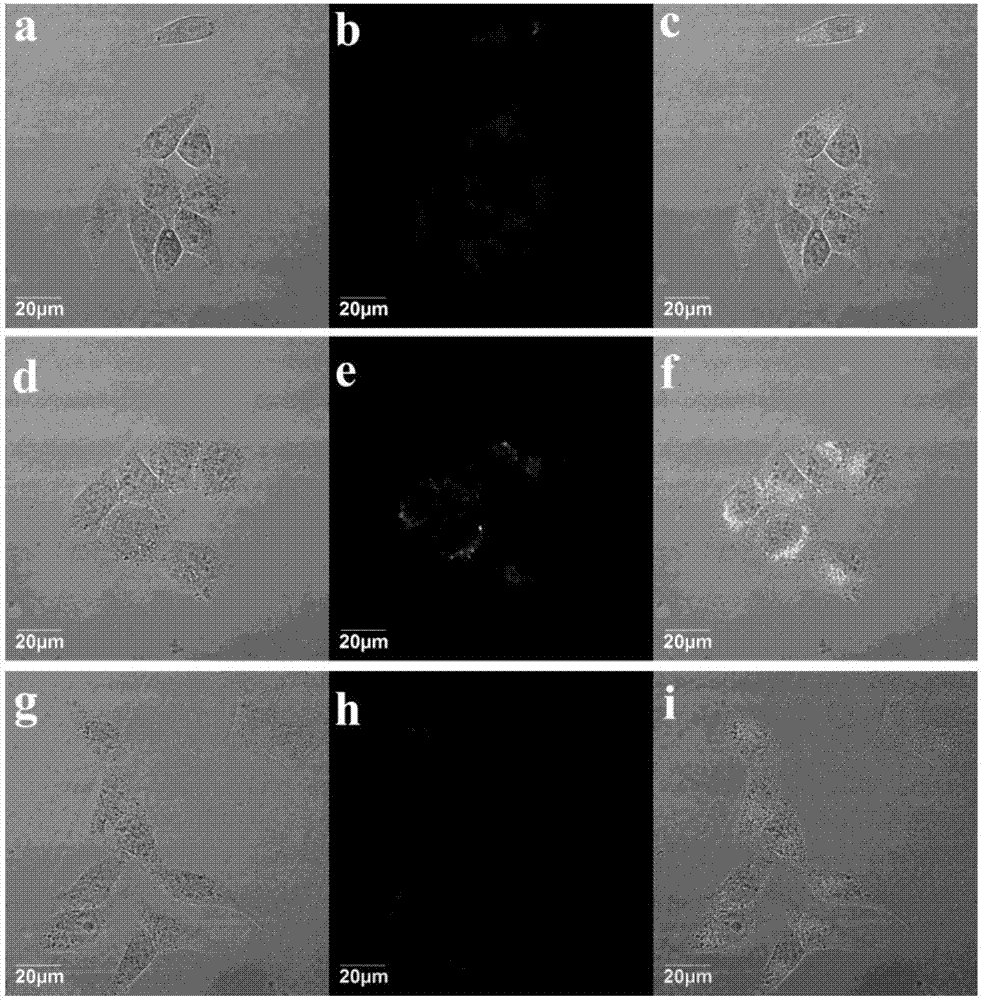
[0037] HeLa cells were respectively immersed in 4 μM compound TPENNO 2 culture medium at 37°C, 5% CO 2 1. After incubating in an incubator with 100% saturated humidity for 20 minutes, pour out the medium, and wash the cells 3 times with fresh medium. Observed under a laser confocal fluorescence microscope, and photographed in bright field and dark field (such as image 3 shown). The results showed that the target compound TPENNO 2 Green fluorescent signal appears in HeLa cells, and under the same conditions, the medium containing 0.5mM Cys was first added to the HeLa cell culture dish and incubated for 24h, and then the target compound TPENNO was added 2 4μM medium was incubated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com