Paclitaxel gastric residence molecular imprinting controlled release administration system and preparation method thereof
A molecular imprinting and gastric retention technology, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of high-sensitivity cardiotoxicity, low solubility, and limited clinical application, etc. To achieve the effect of easy operation and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The gastric retention system is to increase the absorption of drugs, improve bioavailability, and prolong the residence time of drugs in the stomach. In order to investigate the floating performance of the drug, the floating characteristics of the paclitaxel gastric retention controlled sustained-release drug delivery system and the blank control group without liquid crystal monomer and POSS were observed within 24 hours. The specific operation steps are as follows:
[0037] a. Preparation method of molecularly imprinted drug carrier of gastric retention controlled sustained release drug delivery system using paclitaxel as template:
[0038]The formula is as follows: (mass percentage)
[0039] Paclitaxel 1.83%
[0040] Methacrylic acid 0.55%
[0041] Ethylene glycol dimethacrylate 3.17%
[0042] Liquid crystal monomer 4.51%
[0043] Polyhedral oligosilsesquioxane 24.17%
[0044] Azobisisobutyronitrile 0.93%
[0045] Toluene 52.85%
[0046] Acetonitrile 11.99%. ...
Embodiment 2
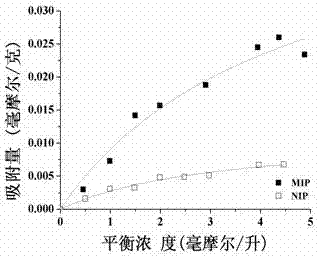
[0055] Paclitaxel equilibrium adsorption experiment was used to study the specific adsorption performance of the new molecularly imprinted polymer with paclitaxel as the template on the template molecule paclitaxel. Adsorption isotherms in the range of 0.5–5 mmol / L. The specific operation steps are as follows:
[0056] a. Synthesize the molecularly imprinted drug carrier MIP of the intragastric retention controlled sustained-release drug delivery system with paclitaxel as the template by the same method as above (Example 1). The non-imprinted polymer NIP does not add the template molecule paclitaxel, and the rest of the steps are the same as the synthesis of MIP.
[0057] b. Weigh 10.0 mg of dry novel paclitaxel molecularly imprinted polymers and non-imprinted polymers containing liquid crystal monomers and POSS, respectively, and put them into 5 mL centrifuge tubes, add 2.0 mL paclitaxel ethanol solution with a concentration of 0.5-5 mmol / L , put it into a shaker (power 100 ...
Embodiment 3
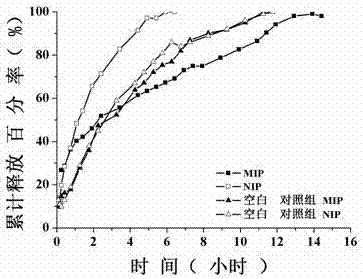
[0064] The drug release experiment in vitro is a model study of the drug release kinetics of the liquid crystal monomer loaded with paclitaxel and the new molecularly imprinted polymer of POSS. In order to investigate the release model of the drug, the total amount of drug released in a certain period of time was measured by the liquid crystal monomer loaded with paclitaxel and the new molecularly imprinted polymer of POSS, the non-imprinted polymer, and the blank control group without liquid crystal and POSS. The specific operation steps are as follows:
[0065] a. Synthesize the molecularly imprinted drug carrier MIP of the intragastric retention controlled sustained release drug delivery system with paclitaxel as the template by the above method (Example 1), and the synthesis of the paclitaxel molecularly imprinted polymer without liquid crystal monomer and POSS in the control group except without adding Except for the liquid crystal monomer and POSS, the other steps are th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com