Self-supporting nickel phosphide catalyst and preparation method and application thereof
A technology of nickel phosphide and catalyst, which is applied in the field of catalysis, can solve the problems of catalyst specific surface area reduction, limitation of industrial application, sintering and agglomeration of copper nanoparticles, and achieve high selectivity of methyl glycolate, significant industrial application value, inhibition The effect of surface agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
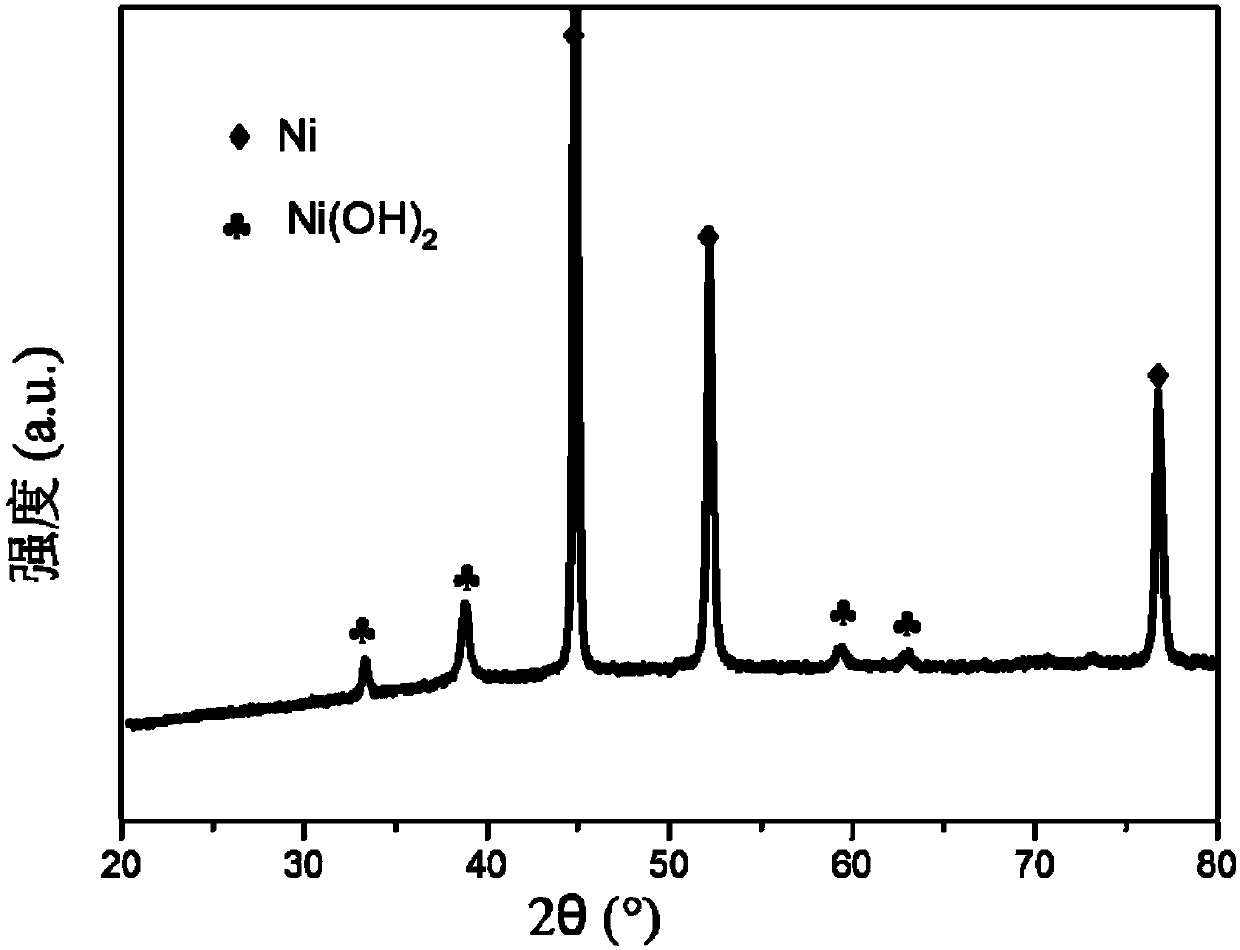
[0059] 1) Weigh 1g of nickel foam, ultrasonically treat it in 20mL of industrial alcohol for 30 minutes, wash it with distilled water and treat it with 1M dilute nitric acid for 2 minutes, wash it with distilled water and immerse it in a hydrothermal reaction kettle containing nickel nitrate and ammonium chloride aqueous solution, The concentration of nickel nitrate is 0.01M, the concentration of ammonium chloride is 0.04M, the hydrothermal temperature is 100°C, and the hydrothermal time is 3 hours. After the reaction is completed, it is washed with distilled water and dried at 100°C. a self-supporting nickel phosphide catalyst precursor grown with a nickel hydroxide crystalline layer;
[0060] 2) Place the prepared self-supporting nickel phosphide catalyst precursor in the reaction tube, after purging with 50mL / min nitrogen for 30 minutes, pass it into the atmosphere of phosphine, and start the temperature-raising phosphating treatment: treat at 300°C 0.5 hour, and finally pu...
Embodiment 2
[0073] 1) with the step 1) of embodiment 1);
[0074] 2) Weigh 1g of the prepared self-supporting nickel phosphide catalyst precursor, impregnate it with an ammonium phosphate aqueous solution (1.5g ammonium phosphate dissolved in 0.7g water) to an equal volume, ultrasonicate for 10 minutes after impregnation, and place at 100°C Oven, dried for 6 hours, and then placed in a reaction tube for hydrogen reduction treatment at 20-800°C: in a mixed atmosphere of hydrogen and nitrogen (hydrogen gas fraction is 10%), the gas flow rate is 50mL / min, at 20-800°C In the temperature range of 250°C, the heating rate is 10°C / min; in the temperature range of 250-350°C, the heating rate is 2°C / min; in the temperature range of 350-800°C, the heating rate is 1°C / min. Free-standing nickel phosphide catalyst.
[0075] Figure 5 It is the X-ray diffraction spectrum figure of the self-supporting nickel phosphide catalyst prepared by the present embodiment, by Figure 5 It can be seen that the se...
Embodiment 3
[0080] 1) with the step 1) of embodiment 1);
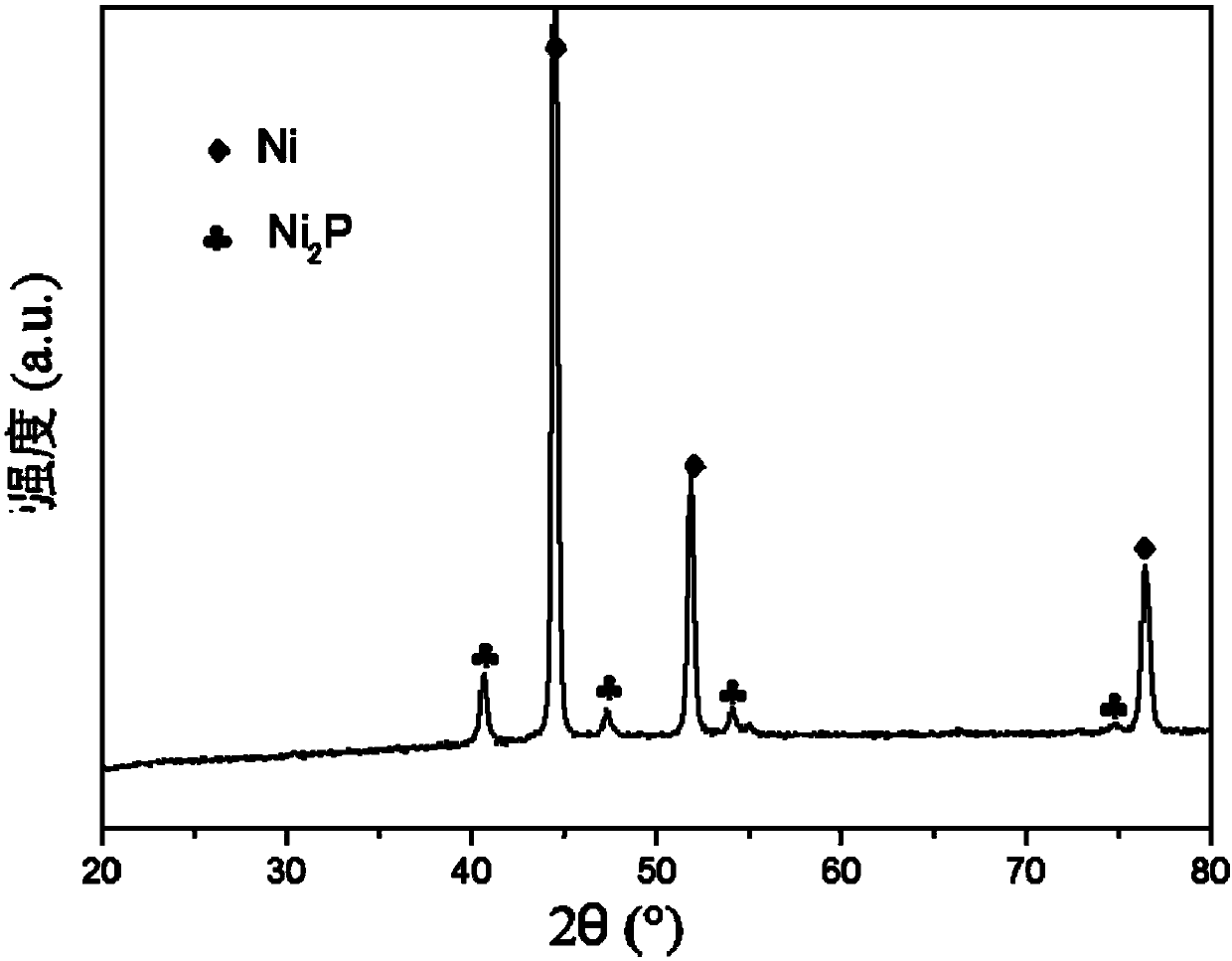
[0081] 2) Weigh 1 g of the prepared self-supporting nickel phosphide catalyst precursor, impregnate it with an ammonium phosphate aqueous solution (1 g of ammonium phosphate dissolved in 0.7 g of water), perform an equal volume impregnation on it, and ultrasonically treat it for 10 minutes after impregnation, and place it in an oven at 100 ° C , dried for 6 hours, and then placed in a reaction tube for temperature-programmed reduction treatment at 20-650°C: the reducing gas is hydrogen, and the hydrogen flow rate is 10mL / min; in the temperature range of 20-250°C, the heating rate is 10°C / min , in the temperature range of 250-350°C, the heating rate is 2°C / min, and in the temperature range of 350-650°C, the heating rate is 1°C / min, to obtain the self-supporting nickel phosphide catalyst.
[0082] Figure 6 It is the X-ray diffraction spectrum figure of the self-supporting nickel phosphide catalyst prepared by the present embodimen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Outer diameter | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com