A kind of betaine type antibacterial finishing agent and preparation method thereof
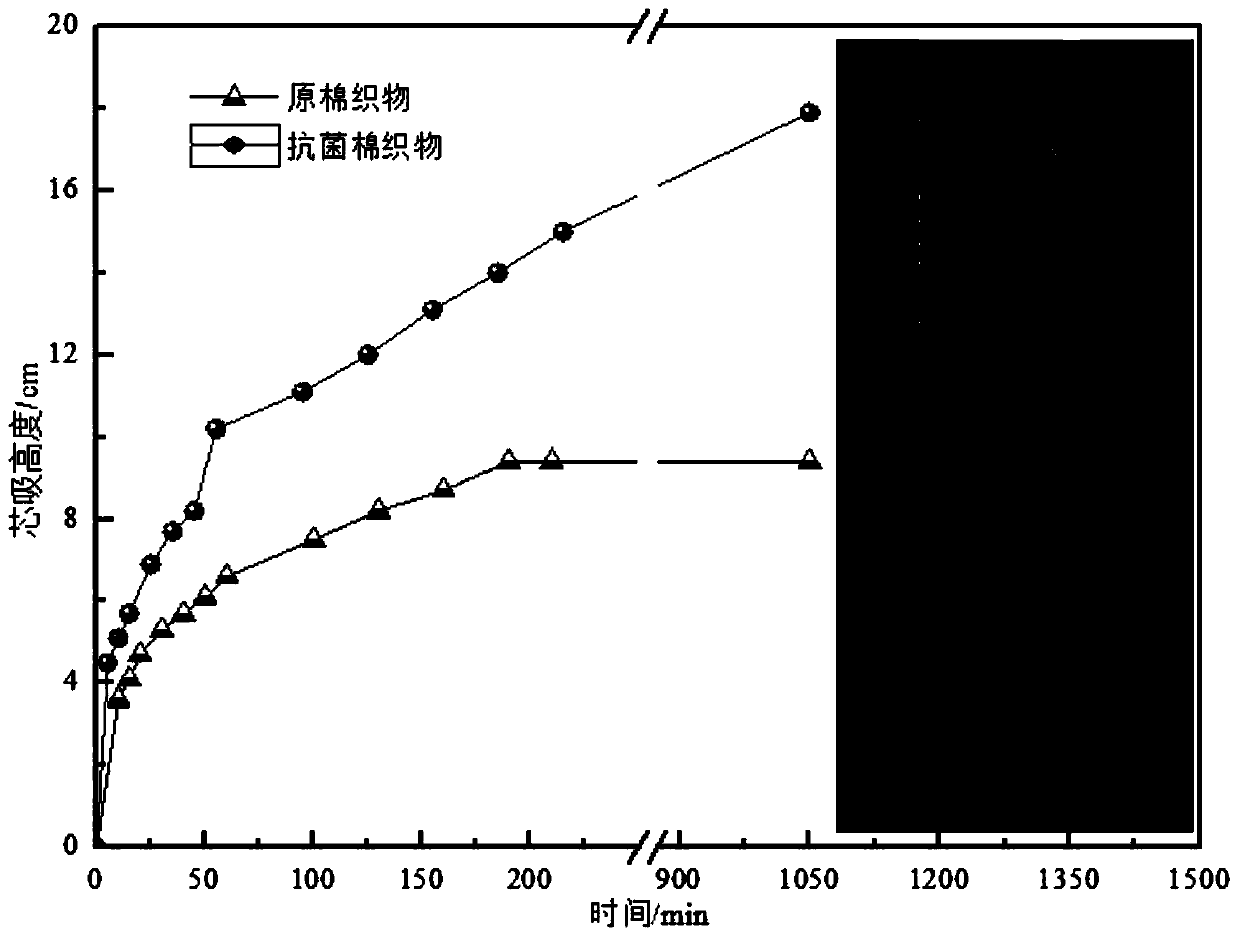
An antibacterial finishing agent and betaine technology, which is applied in the field of betaine antibacterial finishing agent and its preparation, can solve the problems of easy dissolution of quaternary ammonium salts, poor stability of reactive groups, lack of reactive functional groups, etc., and improve air permeability and moisture permeability , improve the hydrophilic performance, and the effect of simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
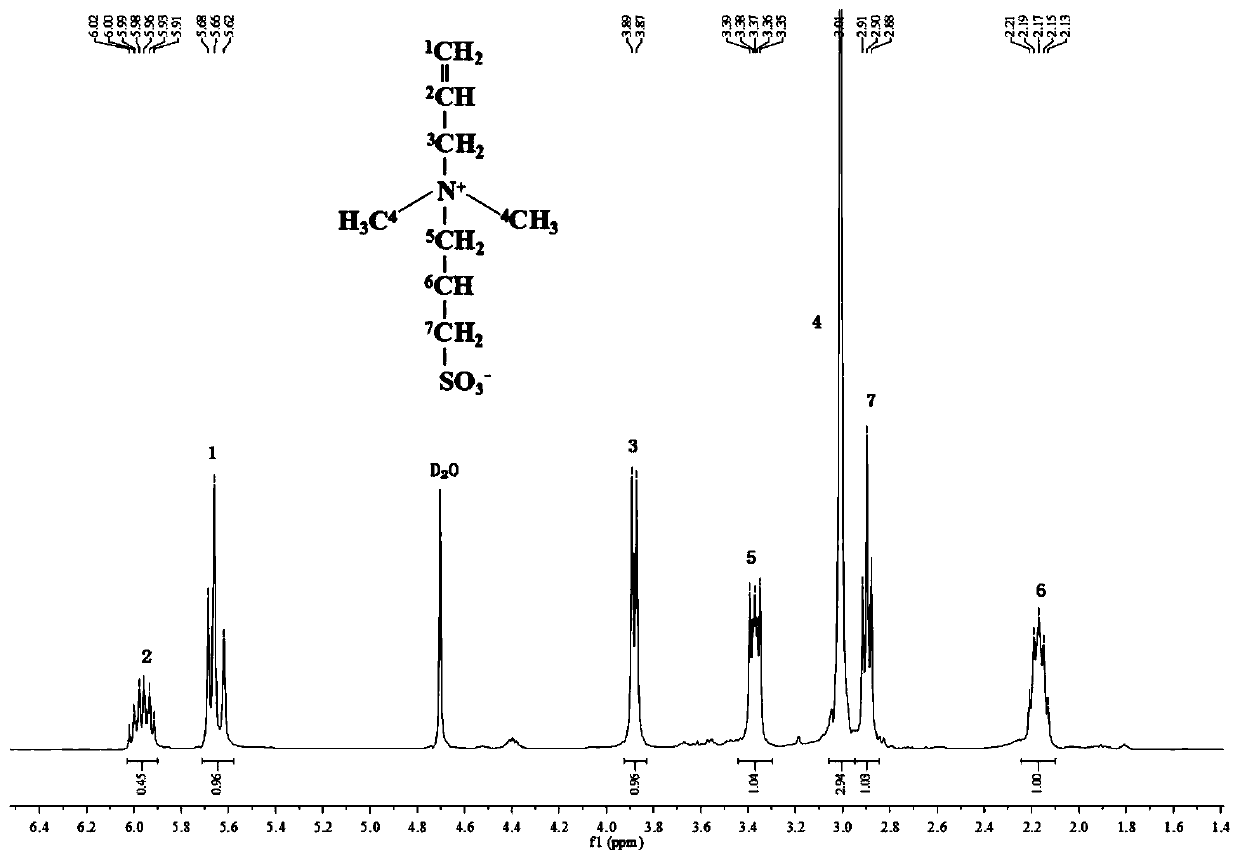
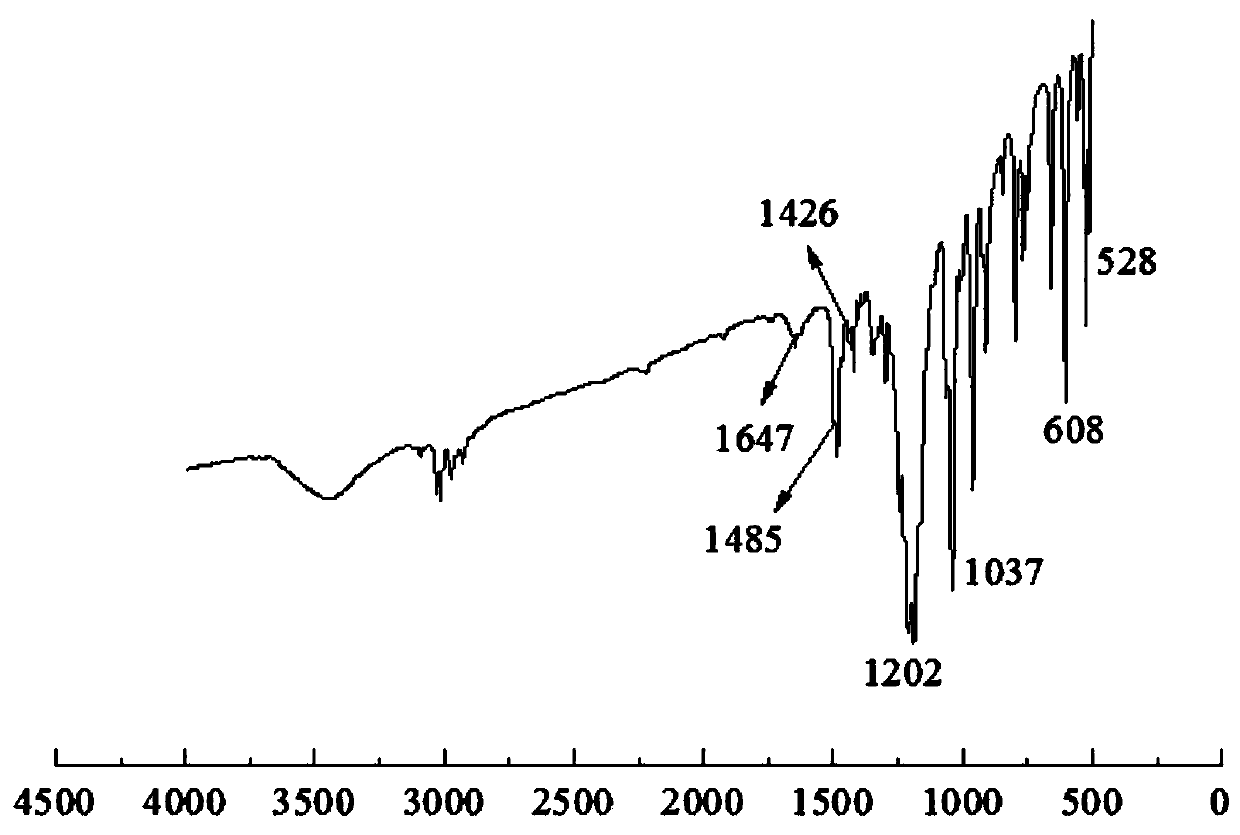
[0039] Weigh 0.6mol (51.1g) dimethylacrylamine【CH 2 =CHCH 2 N(CH 3 ) 2 】Put it into a round-bottomed flask equipped with mechanical stirring, nitrogen inlet tube, condensing reflux tube and thermometer, raise the temperature to 50°C, feed high-purity nitrogen gas for 30 minutes to remove oxygen, and slowly add propane sultone dropwise with a constant pressure funnel【 Abbreviated as 1,3-PS] 0.6mol (73.2g, dissolved in 600mL of absolute ethanol), reacted for 2 hours after the dropwise addition, and obtained a white precipitate, which was purified by rotary evaporation to obtain the product betaine monomer, whose structural formula is CH 2 =CHCH 2 + N(CH 3 ) 2 (CH 2 ) 3 SO 3 — .
[0040] Take by weighing 0.3mol (62.1g) this betaine monomer and 0.3mol (29.4g) maleic anhydride [ Abbreviated as MAH], dissolved in 350mL deionized water, put into a round-bottomed flask equipped with mechanical stirring, nitrogen gas introduction tube, condensing reflux tube and thermomet...
Embodiment 2
[0046] Weigh 0.5mol (56.5g) diethylpropenylamine【CH 2 =CHCH 2 N(CH 2 CH 3 ) 2 】Put it into a round-bottomed flask equipped with mechanical stirring, nitrogen inlet tube, condensing reflux tube and thermometer, raise the temperature to 40°C, feed high-purity nitrogen gas for 30 minutes to remove oxygen, and slowly add 0.5mol (61.1g, Dissolve 1,3-PS in 500mL acetone), react for 5 hours after the dropwise addition, and obtain a white precipitate, which is purified by rotary evaporation to obtain the product betaine monomer, whose structural formula is CH 2 =CHCH 2 + N(CH 2 CH 3 ) 2 (CH 2 ) 3 SO 3 — .
[0047] Weigh 0.5mol (103.5g) of the betaine monomer and 0.5mol (49.0g) of MAH, dissolve them in 600mL of deionized water, and add them into a round bottom flask equipped with mechanical stirring, nitrogen inlet tube, condensing reflux tube and thermometer , pass through high-purity nitrogen for 30 minutes to remove oxygen, raise the temperature to 80°C, slowly add the...
Embodiment 3
[0050] Weigh 0.4mol (34.1g) dimethylacrylamine【CH 2 =CHCH 2 N(CH 3 ) 2 】Put it into a round-bottomed flask equipped with mechanical stirring, nitrogen inlet tube, condensing reflux tube and thermometer, raise the temperature to 30°C, feed high-purity nitrogen gas for 30 minutes to remove oxygen, and slowly add butane sultone dropwise with a constant pressure funnel【 Abbreviated as 1,4-BS] 0.4mol (54.5g, dissolved in 400mL tetrahydrofuran), reacted for 8 hours after the dropwise addition, and obtained a white precipitate, which was purified by rotary evaporation to obtain the product betaine monomer, whose structural formula is CH 2 =CHCH 2 + N(CH 3 ) 2 (CH 2 ) 4 SO 3 — .
[0051] Weigh 0.6mol (124.2g) of the betaine monomer and 0.6mol (58.8g) of MAH, dissolve them in 750mL of deionized water, and add them into a round bottom flask equipped with mechanical stirring, nitrogen inlet tube, condensing reflux tube and thermometer , pass through high-purity nitrogen for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
| whiteness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com