New oxahydrofluoroether compounds and preparation method and application of new oxahydrofluoroether compounds
A technology of compound and hydrofluoroether, applied in the field of novel oxahydrofluoroether compound and its preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
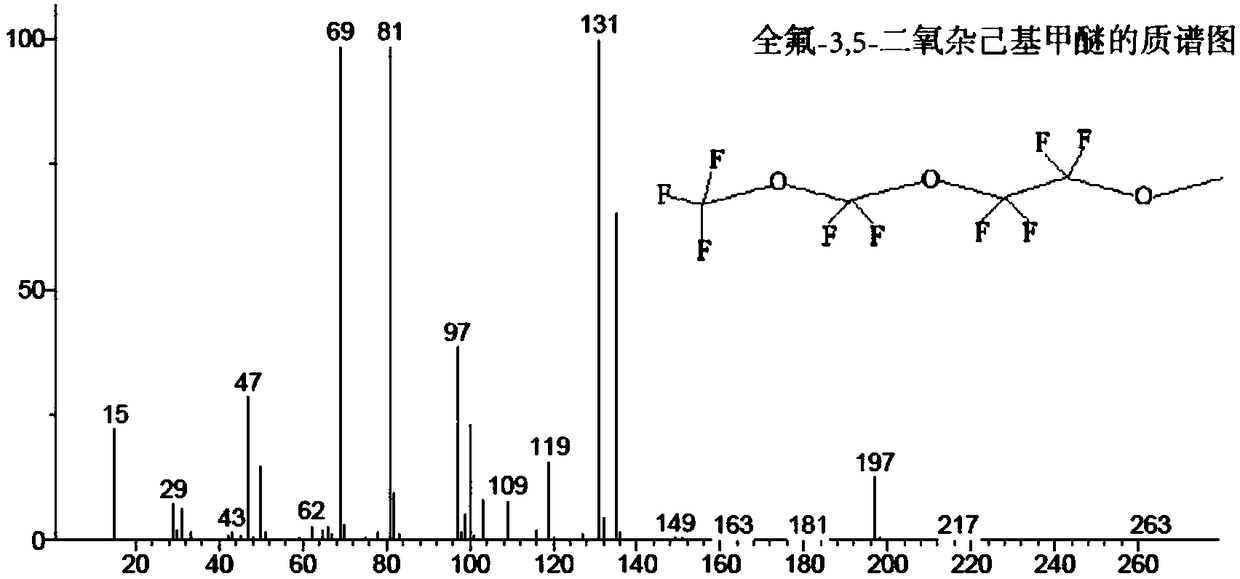
[0100] CF 3 OCF 2 OCF 2 CF 2 OCH 3 Preparation of:
[0101] 2moLCF 3 OCF 2 OCF 2 COF, 2.1moL anhydrous diglyme, 2.4moL potassium fluoride, 0.0072moL phase transfer catalyst tetrabutylammonium chloride, and 2moL dimethyl sulfate were added to a 2L autoclave, and the While stirring at a rotating speed, the temperature was raised to 52°C, and the reaction was carried out at 52°C for 24 hours. After 24 hours of reaction, 200 g of potassium hydroxide aqueous solution with a mass fraction of 50% was added, the temperature was raised to 55°C, and the temperature was kept at 55°C for 45 minutes, and then used A separatory funnel is used for liquid separation, and the liquid in the lower layer is rectified to obtain the target product. The resulting target product was distilled (b.p.=76°C) to obtain the product CF with a purity of 99.5% and a yield of 96.4%. 3 OCF 2 OCF 2 CF 2 OCH 3 .
Embodiment 2
[0103] CF 3 OCF 2 OCF 2 CF 2 OCF 2 CF 2 OCH 3 Preparation of:
[0104] Add 0.516mol potassium fluoride, 0.45mol tetraethylene glycol dimethyl ether to a 2L autoclave, and then add 2.0mol CF 3 OCF 2 OCF 2 COF, reduce the inner temperature of the autoclave to 0°C through a dry ice trap, and then feed 2.0mol hexafluoropropylene oxide at a flow rate of 0.5g / min. After the feeding is completed, continue to stir at a speed of 200 rpm for 30 minutes Addition reaction is carried out, and the crude product is obtained by rectification CF 3 OCF 2 OCF 2 CF 2 OCF (CF 3 ) COF. After gas chromatography analysis, the product CF 3 OCF 2 OCF 2 CF 2 OCF (CF 3 ) The content of COF is 78%.
[0105] 1.0molCF 3 OCF 2 OCF 2 CF 2 OCF (CF 3 ) COF was dropped into a three-necked flask equipped with 1.2 mol of anhydrous sodium carbonate and 1.80 mol of diethylene glycol dimethyl ether with a reflux condenser, and the inner temperature of the reaction was maintained at 50° C. for...
Embodiment 3
[0116] CF 3 OCF 2 OCF 2 CF 2 OCH 3Preparation of:
[0117] 2moLCF 3 OCF 2 OCF 2 COF, 2.1moL anhydrous diglyme, 2.4moL potassium fluoride, 0.0075moL phase transfer catalyst, and 2moL dimethyl sulfate were added to a 2L autoclave, and the temperature was raised to 52 while stirring at a speed of 300 rpm. ℃, keep it warm at 52°C for 24 hours to react, after 24 hours of reaction, add 200g of potassium hydroxide aqueous solution with a mass fraction of 50%, raise the temperature to 55°C, keep it warm at 55°C for 45 minutes, and then use a separatory funnel to separate the liquid , the lower liquid was subjected to rectification to obtain the target product. The resulting target product was distilled (b.p.=76°C) to obtain the product CF with a purity of 99.1% and a yield of 97.1%. 3 OCF 2 OCF 2 CF 2 OCH 3 . The phase transfer catalyst is a mixture of tetrabutylammonium fluoride and tetrabutylammonium chloride in a molar ratio of 1:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com