A kind of nano drug carrier, drug carrier system and preparation method for ultrasonic controlled release based on pullulan
A nano-drug carrier, pullulan polysaccharide technology, applied in non-active ingredients medical preparations, active ingredients-containing medical preparations, drug combinations, etc., can solve drug resistance, reduce the therapeutic effect of cancer cells, tumors Cells can not reach the drug concentration and other problems, to achieve the effect of good water solubility and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 P-OC
[0048] (1) Under nitrogen protection, in a 50mL round bottom flask, HTEMPO (1.034g, 6mmoL) and triethylamine (0.83mL) were dissolved in 15mL of anhydrous dichloromethane and stirred in ice bath for 30min, then the stearin Acyl chloride (1.81mL, 6mmoL) was dissolved in 10mL of anhydrous dichloromethane, slowly added, and stirred at 30°C for 30h, the reaction mixture was poured into a separatory funnel, extracted with a large amount of deionized water, and unreacted HTEMPO was washed away. , take out the organic phase, dry with anhydrous magnesium sulfate, filter, and remove dichloromethane by rotary evaporation. The residue is separated from impurities by column chromatography, and eluted with a 9:1 eluent of petroleum ether and ethyl acetate to obtain a red The oily product, piperidinium stearate (abbreviated as OC), was stored in a refrigerator at 4°C.
[0049] (2) Under nitrogen protection, in a 100mL round bottom flask, put pul...
Embodiment 2
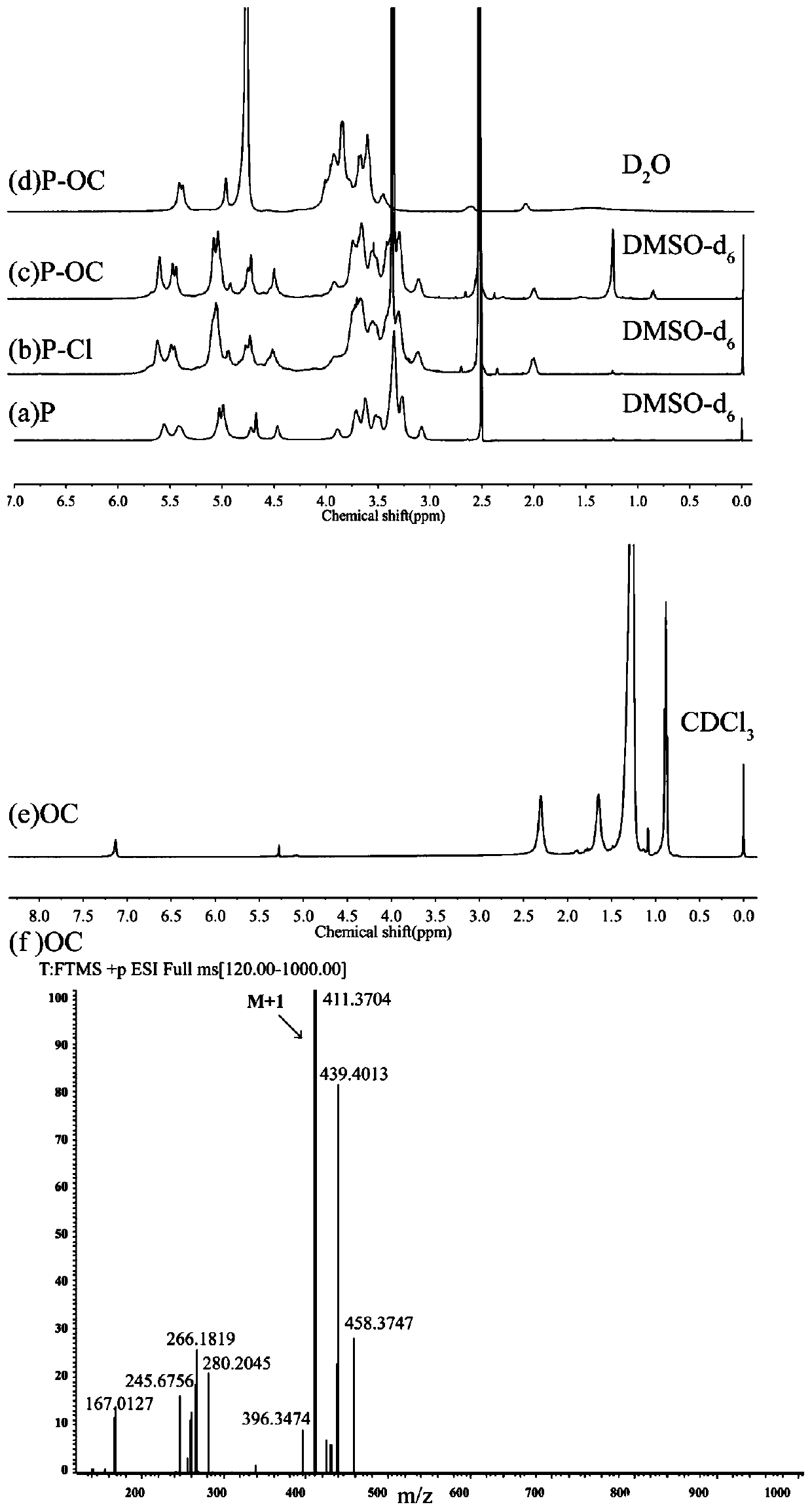
[0053] Example 2 Characterization of Proton Spectrum and High Resolution Mass Spectrometry
[0054] The OC prepared in Example 1 was dissolved in deuterated chloroform and P-Cl, P-OC, and P were dissolved in deuterated dimethyl sulfoxide or deuterated water, and the NMR ( 1 H NMR) for structural verification, and the OC prepared in Example 1 was dissolved in dichloromethane to further verify the structure by high-resolution mass spectrometry.
[0055] The result is as figure 1 Shown, piperidinium stearate (e, CDCl 3 ) in the spectrum, a proton peak appears at δ7.13ppm, which is a characteristic peak formed by the nitroxide free radical on piperidine combined with a proton, and a piperidine methine (-CH-O) proton peak appears at δ5.23ppm, and δ1.26ppm For the four methyl groups on piperidine (CH 3 ) peak, and methyl and methylene proton peaks appear on stearic acid at δ0.88ppm, consistent with its piperidine stearate cleavage peak. Simultaneously the high resolution mass sp...
Embodiment 3
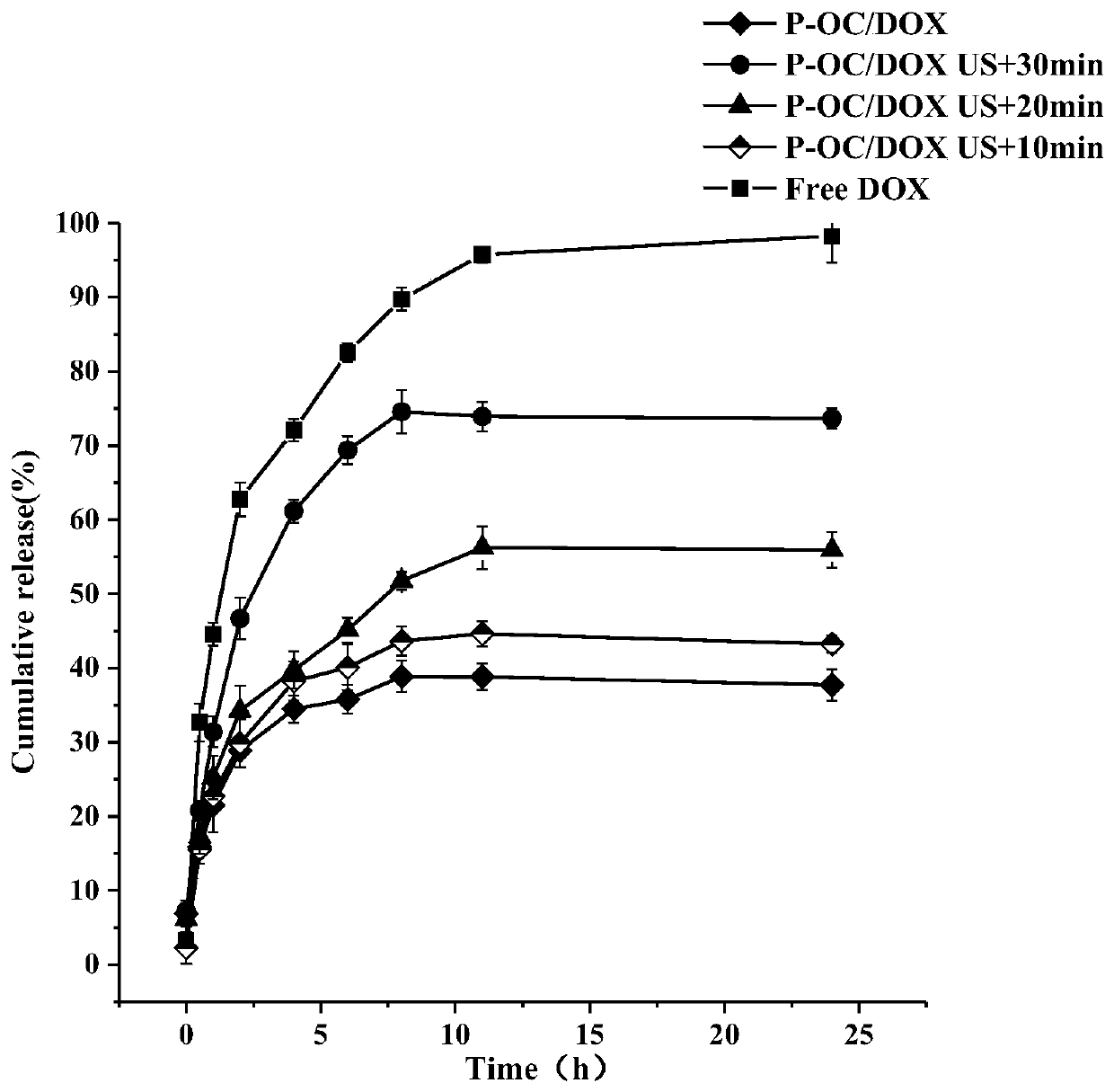
[0056] Example 3 Preparation of P-OC / DOX drug-loaded nanomicelles
[0057] 20 mg of P-OC prepared in Example 1 was dissolved in 4 mL of DMSO, and a mixed solution formed by dissolving 2.1 mg of doxorubicin hydrochloride and 1.03 μL of triethylamine in 400 μL of DMSO was added (wherein, doxorubicin hydrochloride and triethylamine The molar ratio of doxorubicin is 1:2, the concentration of doxorubicin is 5mg / mL, the mixing mass ratio of doxorubicin and P-OC is 1:10), and stirred for 1h, dialyzed for 72h, and P-OC can be obtained after lyophilization / DOX drug-loaded nanomicelles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com