Preparation method and application of iprodione hapten and antigen
A technology for isobacteride and hapten, which is applied in the field of preparation of isobacteride hapten and antigen, can solve the problems of inability to carry out large-scale detection on site, difficult to popularize, and cumbersome to handle, and achieves rapid detection, good affinity, and crossover. The effect of low response rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Example 1 Preparation of iprodione hapten
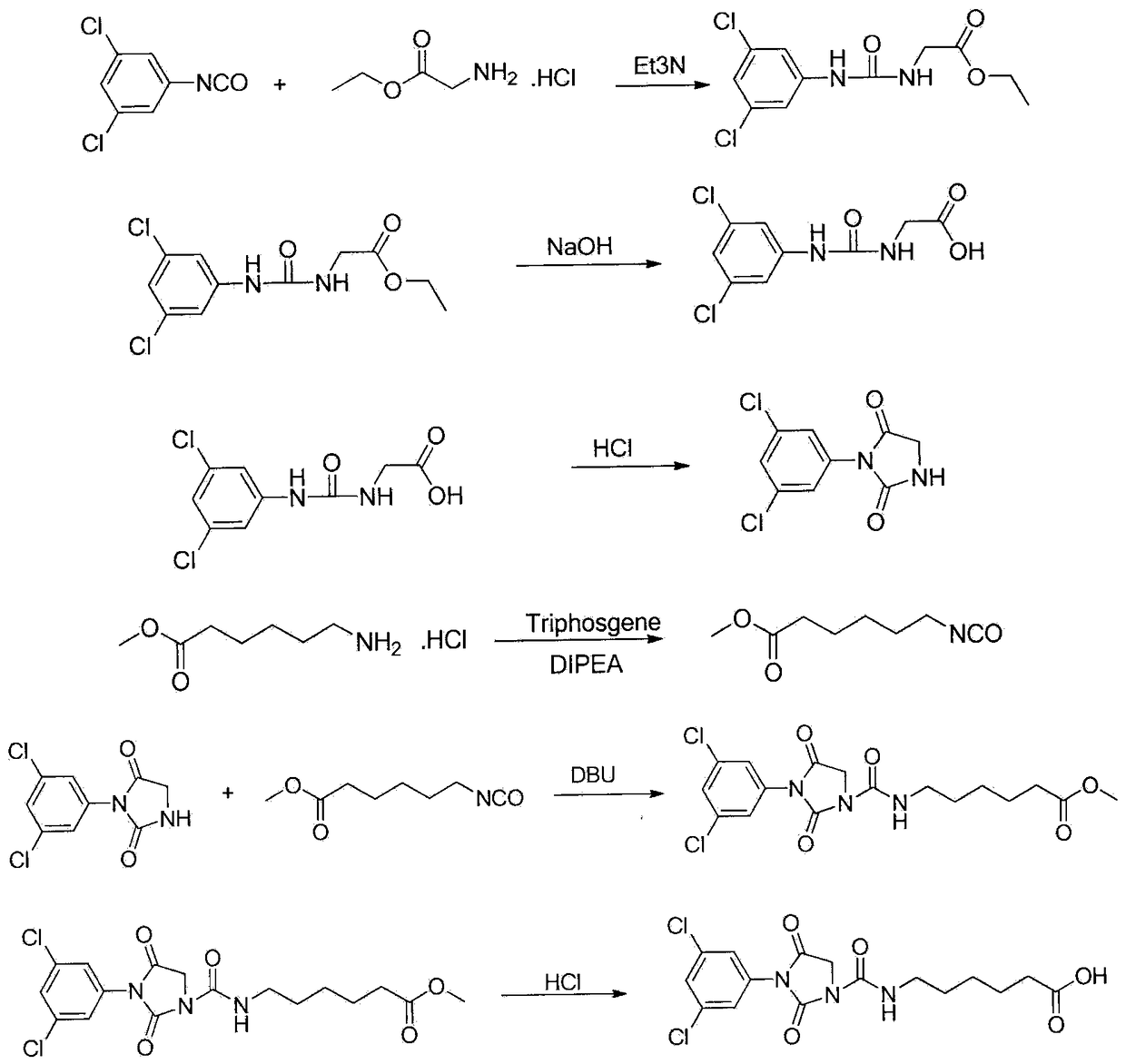
[0030] 1. Synthesis of iprodione hapten (synthetic route see figure 1 )
[0031] 1) Add 8.37 g (60 mmol) ethyl glycine hydrochloride and 16.5 mL (120 mmol) triethylamine into 100 mL dichloromethane, add 10 g (53 mmol) 3,5-dichloromethane dropwise at 0°C Dichloromethane solution of phenylisocyanate, stirred at room temperature overnight, filtered to remove insoluble solids, the filtrate was washed with 2 N hydrochloric acid, saturated sodium bicarbonate solution, and saturated brine, and the organic layer was dried with anhydrous magnesium sulfate. After distilling off the solvent, a white Solid ethyl 2-(3-(1,5-dichlorophenylureido))acetate.
[0032] 2) Add the above white solid into 150 mL of 6% sodium hydroxide aqueous solution, heat to 90°C for 3 hours under stirring, cool to room temperature, extract a small amount of unreacted raw materials and by-products with ethyl acetate, and use the water phase at 0°C The pH was adj...
Embodiment 2
[0040] Example 2 Preparation of iprodione antigen
[0041] 1. Synthesis of Iprodione Immunogen
[0042] Iprodione hapten is conjugated with bovine serum albumin (BSA) to obtain immunogen.
[0043] Take 9.0 mg of iprodione hapten, dissolve it in 1.0 mL of dimethylformamide (DMF), add 0.18 mL of isobutyl chloroformate, add 0.3 mL of pyridine, and stir at room temperature for 5 h to obtain hapten activation solution A; Take 50 mg of bovine serum albumin (BSA) and fully dissolve it in 3.8 mL of phosphate buffered saline (PBS) to obtain liquid B. Add liquid A to liquid B dropwise, stir at room temperature for 5 h, and add 0.01 mol / L PBS at 4 °C for 3 days to remove unreacted small molecular substances to obtain iprodione-BSA immunogen; store at -20 °C for future use.
[0044] 2. Synthesis of iprodione coating agent
[0045] Iprodione hapten was coupled with ovalbumin (OVA) to obtain the coating source.
[0046] Take 7.0 mg of iprodione hapten, dissolve it in 1.0 mL of DMF, add ...
Embodiment 3
[0055] Example 3 Preparation of iprodione monoclonal antibody
[0056] 1. Obtaining hybridoma cells
[0057] 1) First immunization: fully emulsify the iprodione hapten-BSA conjugate (immunogen) with the same amount of complete Freund's adjuvant, and subcutaneously inject 6-week-old Balb / c mice at an immunization dose of 150 μg / Only;
[0058] 2) Booster immunization twice: from the first immunization, booster immunization once every two weeks, with Freund's incomplete adjuvant instead of Freund's complete adjuvant, the method and dosage are the same as the first immunization;
[0059] 3) One week after the last booster immunization, the fundus vein blood was collected to measure the titer and inhibition. When there was inhibition and the titer reached more than 1:10000, the following last immunization was carried out: intraperitoneal injection of 0.1 mL of the immunogen solution without any adjuvant, and then executed three days later Mice, whose spleen was fused with myelom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com