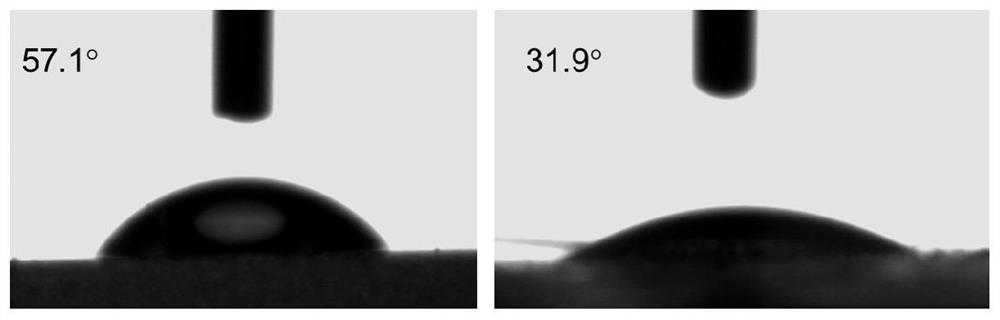
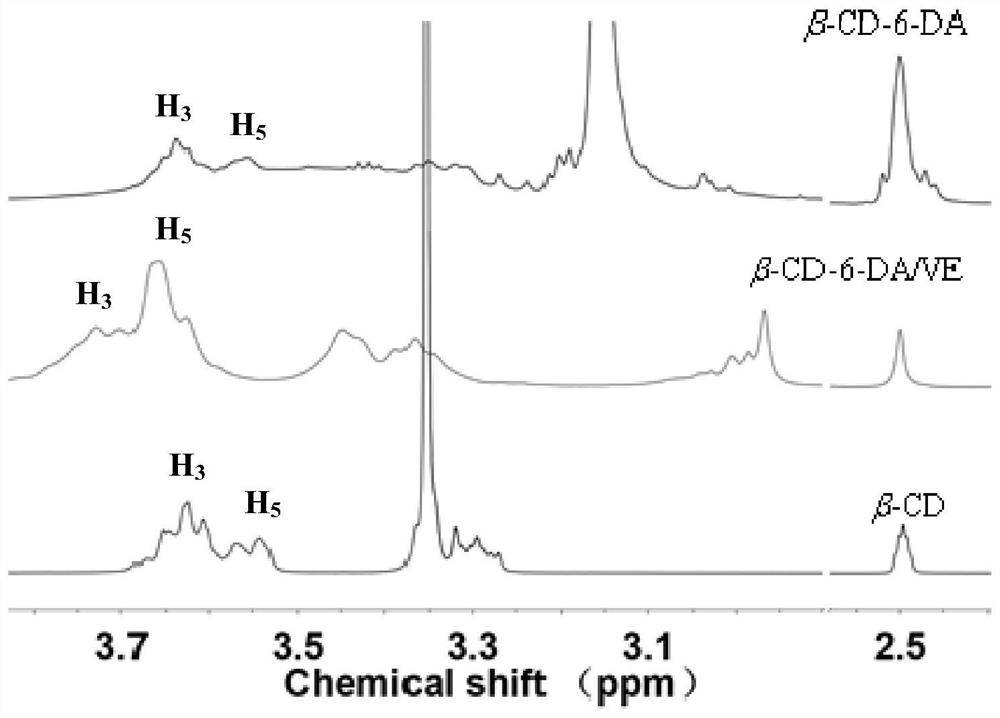
A kind of synthetic method of dopamine derivative modified by cyclodextrin
A synthesis method and cyclodextrin technology, which is applied in the direction of pharmaceutical formulations, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problem of low drug loading, easy uneven deposition, difficult to achieve drug immobilization, controlled release or Sustained release and other issues to achieve the effects of slowing down the polymerization rate, weakening uneven sedimentation, and achieving degradable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of mono-6-deoxy-dopamine cyclodextrin (β-CD-6-DA):
[0030] Dissolve β-cyclodextrin (β-CD) (17.0g, 15.0mmol) in 200mL of 1% NaOH solution, and add 15mL of p-toluenesulfonyl chloride (4.0g, 22.5mmol) dropwise at 0°C ) in acetonitrile solution, stirred and reacted at 25°C for 2h, then filtered, adjusted to pH 2 with hydrochloric acid, kept at 4°C overnight, a large amount of precipitates precipitated, filtered, and the solid was recrystallized 3 times to obtain a white solid, dried in vacuo at 40°C for 5h, to obtain Mono 6-p-toluenesulfonyl cyclodextrin, spare.
[0031] Take 1.00g (0.78mmol) of mono-6-p-toluenesulfonyl cyclodextrin, 0.80g (4.22mmol) of dopamine hydrochloride, add catalyst DMAP (4-dimethylaminopyridine) to 0.19g (1.5mmol), KI (potassium iodide) was 0.06g (0.4mmol), repeatedly evacuated and filled with nitrogen, then injected 10mL of anhydrous DMF (N,N-dimethylformamide) and 1mL of anhydrous methanol into the needle, dissolved the solid sample un...
Embodiment 2
[0034]Take 1.30g (1mmol) of mono-6-p-toluenesulfonyl cyclodextrin, 1.9g (10mmol) of dopamine hydrochloride, add catalyst DMAP to 0.22g (1.8mmol), KI to 0.06g (0.4mmol), repeatedly vacuumize and fill Nitrogen, then inject 13mL of anhydrous DMF and 1.3mL of anhydrous methanol into the needle, dissolve the solid sample under stirring, adjust the pH to 6.8, react at 100°C for 12h, concentrate the obtained reaction mixture, dry silica gel powder column, the eluent volume ratio is n-butanol: acetic acid: water = 12:1:1, and the cyclodextrin-modified dopamine derivative (β-CD-6-DA) is obtained by column chromatography elution, producing The rate is 78.9%.
[0035] (1) On the basis of Example 2, explore the influence of the reaction molar ratio of mono-6-p-toluenesulfonyl cyclodextrin and dopamine hydrochloride on the yield of the reaction product. The results are shown in Table 1.
[0036] The influence of table 1 reaction system substrate molar ratio on product yield
[0037]
...
Embodiment 3
[0048] Oxidative self-polymerization of β-CD-6-DA:
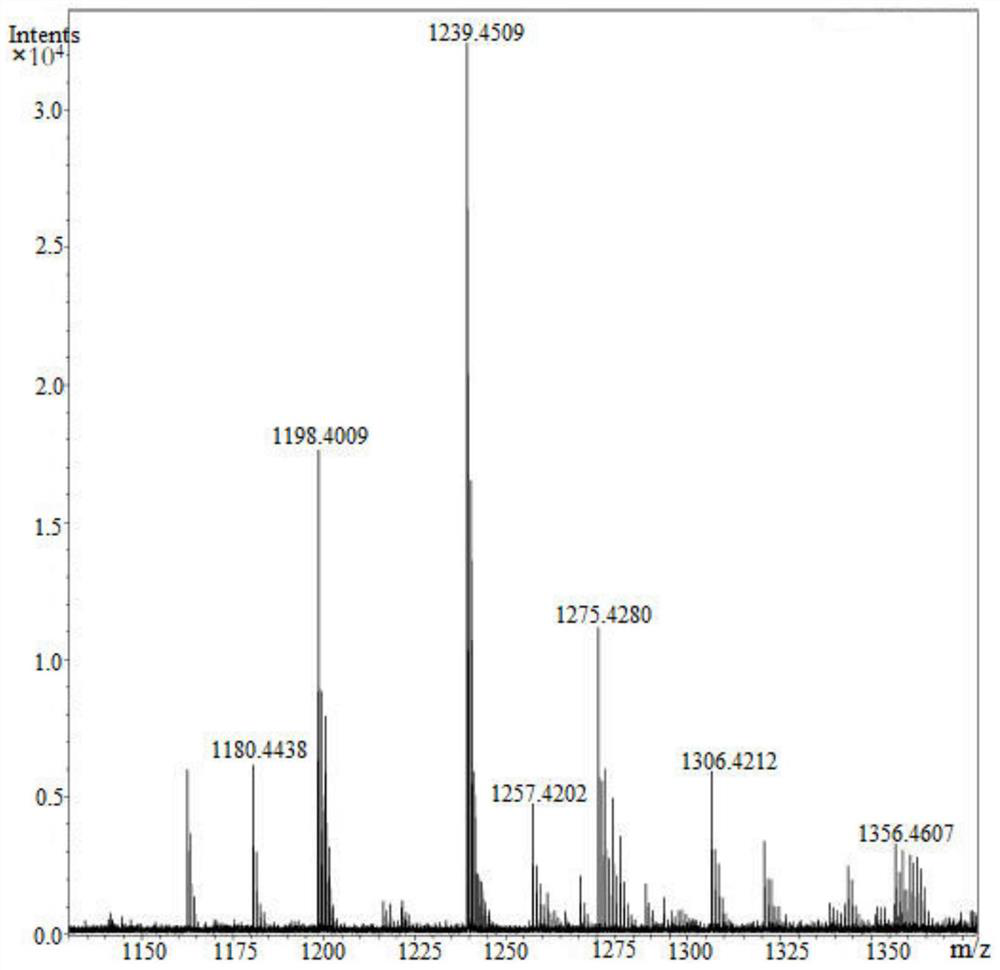
[0049] Take 0.5 g of β-CD-6-DA prepared in Example 2, place it in a weakly alkaline aqueous solution with pH 8.5, stir it in the air for 48 hours, then distill it under reduced pressure to remove water, freeze-dry it, and test the high-resolution mass spectrum, see attached figure 1 . from figure 1 It can be seen that the molecular mass of 1308.3 of the original β-CD-6-DA has disappeared, and the system mainly presents the proton peak with a molecular weight of 1306.4212. This is because the two phenolic hydroxyl groups on β-CD-6-DA are oxidized to two The molecular weight reduction caused by quinone further proves that after dopamine is modified by large molecular weight cyclodextrin, in an alkaline environment, dopamine is not generated as a large amount of polydopamine particles as reported in the literature, but only phenolic hydroxyl groups are oxidized. That is, excessive oxidation of β-CD-6-DA did not occur.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com