Caproic acid bacterium proliferation culture medium and caproic acid fermentation and caproic acid bacterium screening method
A technology of proliferation medium and fermentation method, which is applied in the fields of microbial fermentation and functional bacteria screening and identification, can solve the problems of quantitative detection error, negative influence of color development effect, easy to contaminate miscellaneous bacteria, etc., so as to reduce the toxic effect and improve bacteria. The effect of density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
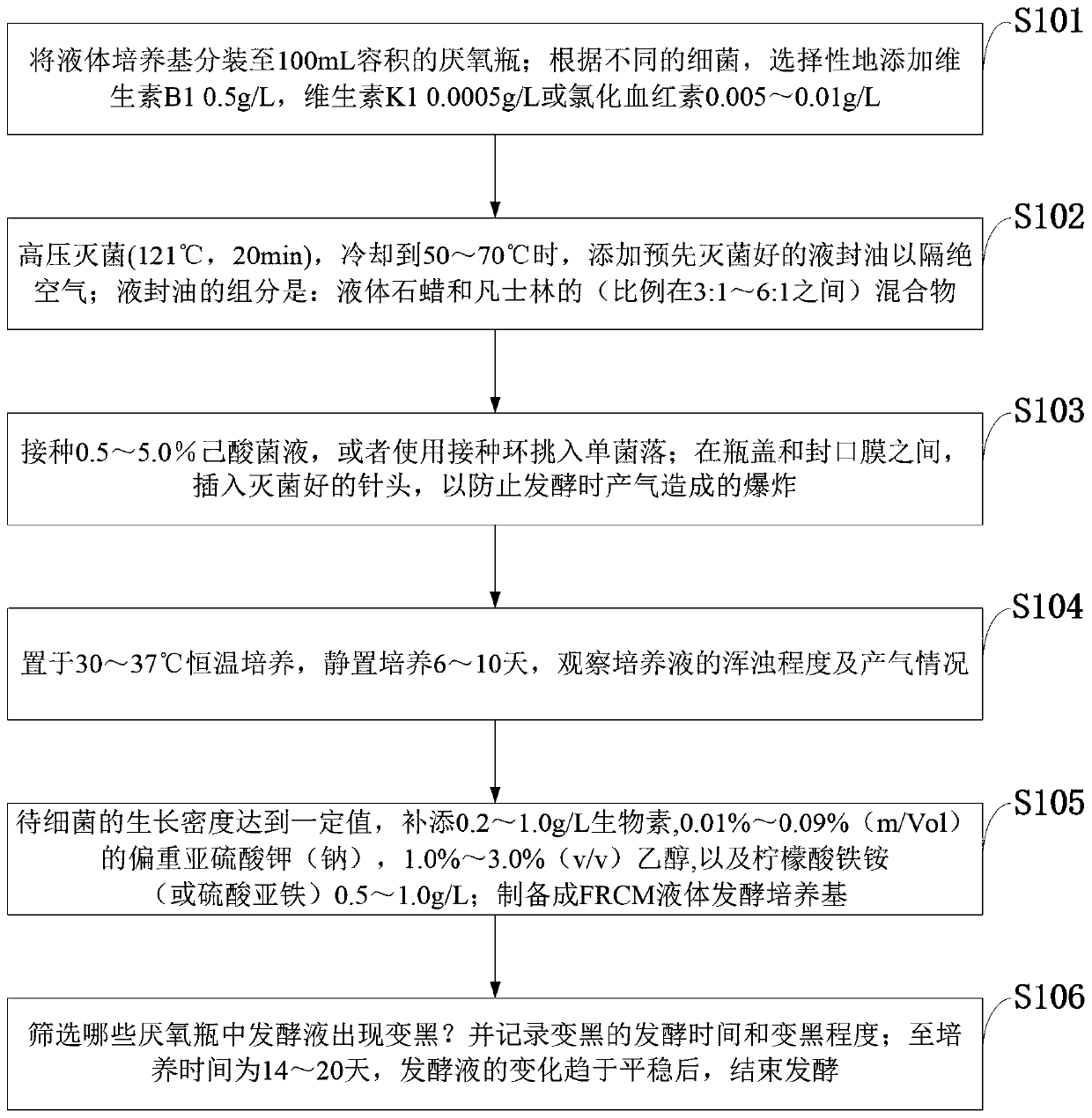
[0079] The proliferation, fermentation and detection methods of the hexanoic acid screening system provided in the embodiments of the present invention specifically include:
[0080] Step 1, preparing LRCM medium (containing ascorbic acid). The ingredients of LRCM liquid medium are: peptone 10.0g / L, beef powder 10.0g / L, yeast powder 3.0g / L, glucose 5.0g / L, soluble starch 1.0g / L, sodium chloride 5.0g / L, sodium acetate 3.0g / L, L-cysteine hydrochloride 0.5g / L, ascorbic acid 0.3g / L, agar 0.5g / L, distilled water 1000mL, pH 6.8±0.1. Divide the liquid medium into 100mL anaerobic bottles.
[0081] Step 2: Autoclave (121°C, 20min), and when cooled to 50-55°C, add pre-sterilized liquid seal oil to isolate the air (see figure 2 a). Liquid seal oil components: liquid paraffin and petrolatum (ratio 3:1).
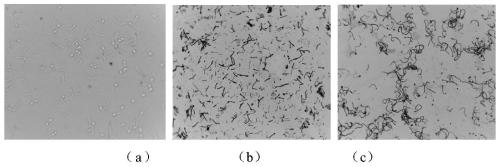
[0082] Step 3, inoculate 1mL of the YL-19 culture solution of the bacterial strain to be tested (see image 3 a). Between the bottle cap and the parafilm, insert a sterilized ne...
Embodiment 2
[0089] The proliferation, fermentation and detection methods of the hexanoic acid screening system provided in the embodiments of the present invention include:
[0090]Step 1, prepare 100 mL of LRCM medium. The ingredients of LRCM liquid medium are: peptone 10.0g / L, beef powder 10.0g / L, yeast powder 3.0g / L, glucose 1.0g / L, soluble starch 1.0g / L, sodium chloride 5.0g / L, sodium acetate 5.0g / L, L-cysteine hydrochloride 0.5g / L, ascorbic acid 1.0g / L, agar 1.0g / L, distilled water 1000mL, pH 6.8±0.1. Divide the liquid medium into 100mL anaerobic bottles.
[0091] Step 2: Autoclave (121°C, 20min), and when cooled to 60-70°C, add vitamin B10.5g / L and hemin 0.01g / L. Add pre-sterilized liquid seal oil to isolate air. The components of liquid seal oil are: liquid paraffin and vaseline (5:1).
[0092] Step 3, inoculate 2.0 mL of the culture solution of the strain XL-48a to be tested. Insert a sterilized needle between the bottle cap and the parafilm to prevent explosions caused by ...
Embodiment 3
[0099] The proliferation, fermentation and detection methods of the hexanoic acid screening system provided in the embodiments of the present invention include:
[0100] Step 1, prepare 11.5L of LRCM liquid medium. LRCM medium components are: peptone 10.0g / L, beef powder 10.0g / L, yeast powder 3.0g / L, glucose 5.0g / L, soluble starch 1.0g / L, sodium chloride 5.0g / L, sodium acetate 6.0 g / L, L-cysteine hydrochloride 0.5g / L, ascorbic acid 0.5g / L, carrageenan 1.0g / L, distilled water 1000mL, pH 6.8±0.1. The liquid medium was divided into 115 anaerobic bottles with a volume of 100 mL.
[0101] Step 2, autoclaving (121°C, 20min), and when cooling to 50-70°C, add pre-sterilized liquid sealing oil to isolate air. The liquid seal oil components are: liquid paraffin and petrolatum (6:1).
[0102] Step 3, use the inoculation loop to pick XL-34a caproic acid bacteria (see image 3 b, 3c) and other single colonies, a total of 115 fermentation systems were prepared (see figure 2 a). Bet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com