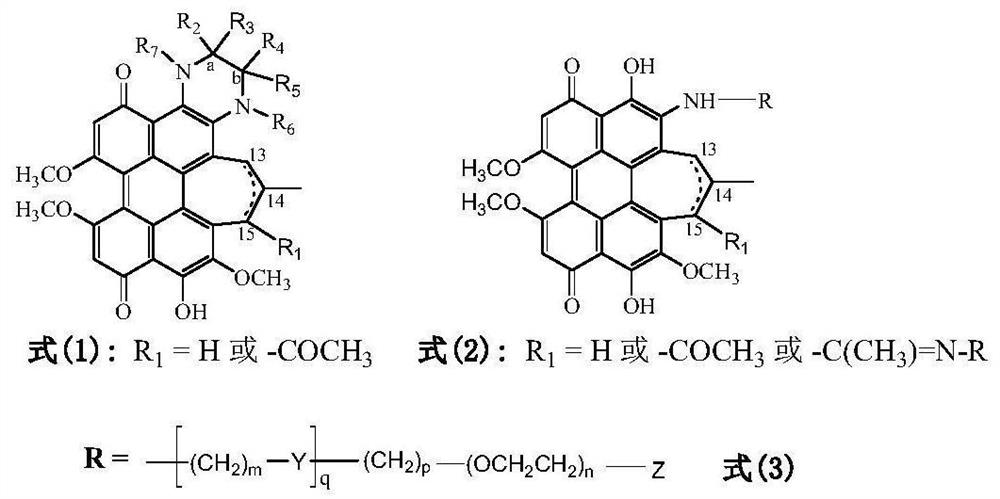
Lipid-water amphiphilic hypocrellin derivative, preparation method and application thereof
A technology of hypocrellin and its derivatives, which can be used in drug combinations, pharmaceutical formulations, photodynamic therapy, etc., and can solve problems such as blood vessel blockage, low solubility, and low cell uptake rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Extraction of Hypocretin A (HA): 100g of Hypocretin A (HA) was pulverized with a pulverizer, placed in a Soxhlet extractor, and continuously extracted for one day with 1000mL acetone as a solvent until the extract was nearly colorless, and the extract was filtered to remove a small amount of infiltrated The solid insoluble matter was then spin-dried to remove acetone, dissolved with 500mL dichloromethane, washed with 4×400mL distilled water, the organic layer was separated and spin-dried, the solid residue was washed with 5×100mL petroleum ether, the solid was spontaneously ignited and air-dried in the air, and then Recrystallize twice with chloroform-petroleum ether, and the obtained crystal is the target product Hypocretin A (HA), with a purity of more than 98%. Thin-layer silica gel plate chromatography, using petroleum ether: ethyl acetate: absolute ethanol (30:10:1) as a developing solvent, can be further purified to obtain high-purity hypocrellin A.
[0076] Prepa...
Embodiment 2
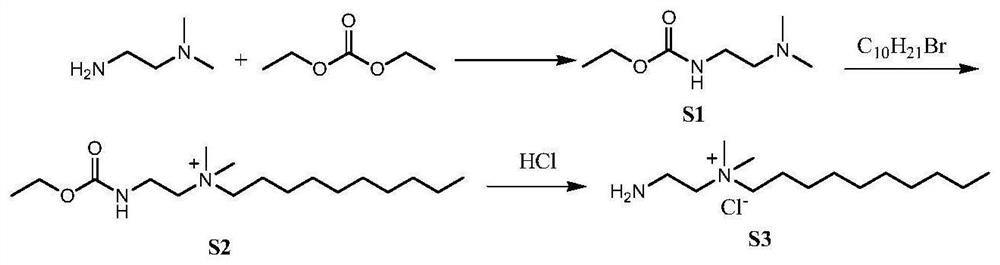
[0079] The present invention contains long-chain quaternary ammonium salt derivatives and is prepared by the following general method, with H 2 NCH 2 CH 2 -N + (CH 3 ) 2 (C 10 h 21 ) for the column description.
[0080]
[0081] Preparation of intermediate S1: N,N-dimethylethylenediamine (4.4g, 0.05mol) and diethyl carbonate (7.10g, 0.06mol) were mixed in a 100ml round bottom flask, and the reaction solution was reacted at 70°C 48h, and then distilled under reduced pressure to obtain 7.20 g of a light yellow liquid with a yield of 89%. 1 H NMR (CDCl 3 ,δ,ppm):5.45(s,-NH-,1H),4.10(d,J=6.5Hz,-CH 2 O,2H),3.24(s,-NH-CH 2 -,2H),2.39(m,-CH 2 N,2H),2.22(d,J=1.5Hz,CH 3 NCH 3 ,6H),1.23(t,J=6.5Hz,-CH 2 CH 3 ,3H).
[0082] Preparation of Intermediate S2: Intermediate S1 and 1-bromodecane (15.25g, 0.05mol) were reacted at 100°C for 48h and then reacted for 72h. The crude product was treated with acetone-diethyl ether (1:1) to obtain 15.83 g of white crystal 2, with a y...
Embodiment 3
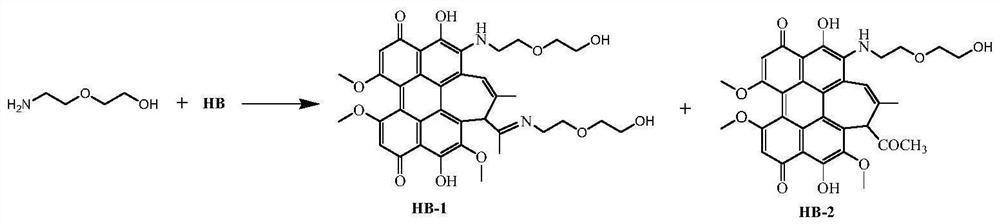
[0085] Hypocretin derivatives modified with aminoethyl glycol (R=-CH 2 CH 2 OCH 2 CH 2 OH) preparation: the synthetic route is attached Figure 4 Shown:
[0086] Dissolve Hypocretin B HB (100mg, 0.18mmol) and aminoethyl glycol (0.40g, 4mmol) in 20mL of anhydrous acetonitrile, mix well, heat to 50°C under nitrogen protection, and stir to react in the dark After 10 h, the reaction was completed, and the solvent was removed by rotary evaporation. The blue-black solid residue was dissolved in 200 mL of dichloromethane, washed once with 100 mL of dilute hydrochloric acid aqueous solution, and washed twice with distilled water. The organic layer was dried with anhydrous magnesium sulfate, filtered, and the organic phase was spin-dried to obtain a crude product. The resulting crude product is further separated by silica gel plate chromatography, and the developing agent is acetone: ethyl acetate (volume ratio is 1:1), and two kinds of blue-black solid products are obtained respe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com