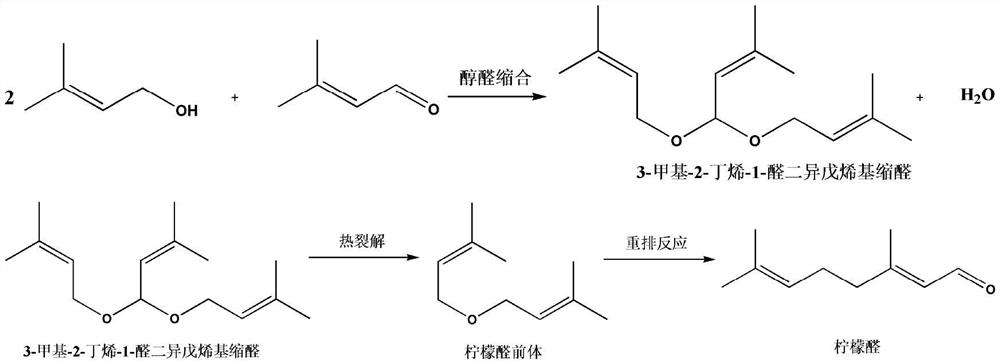
Composite catalyst, preparation method thereof and application thereof in citral synthesis
A composite catalyst, citral technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. The problem of high reaction temperature can reduce the formation of high boiling point substances, improve the space effect, and simplify the preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
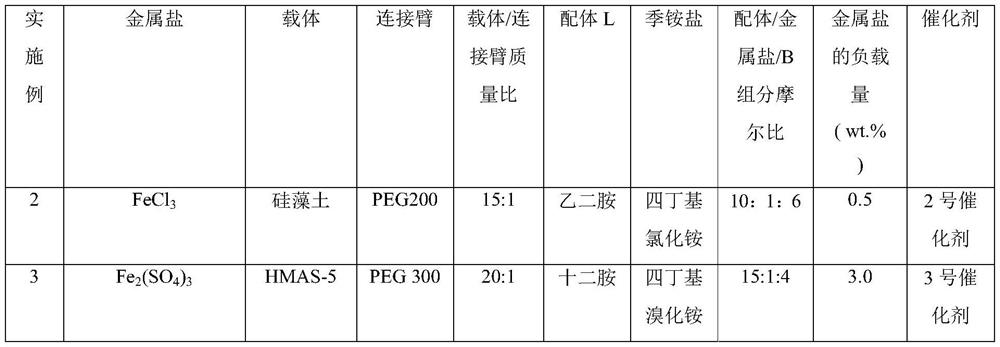
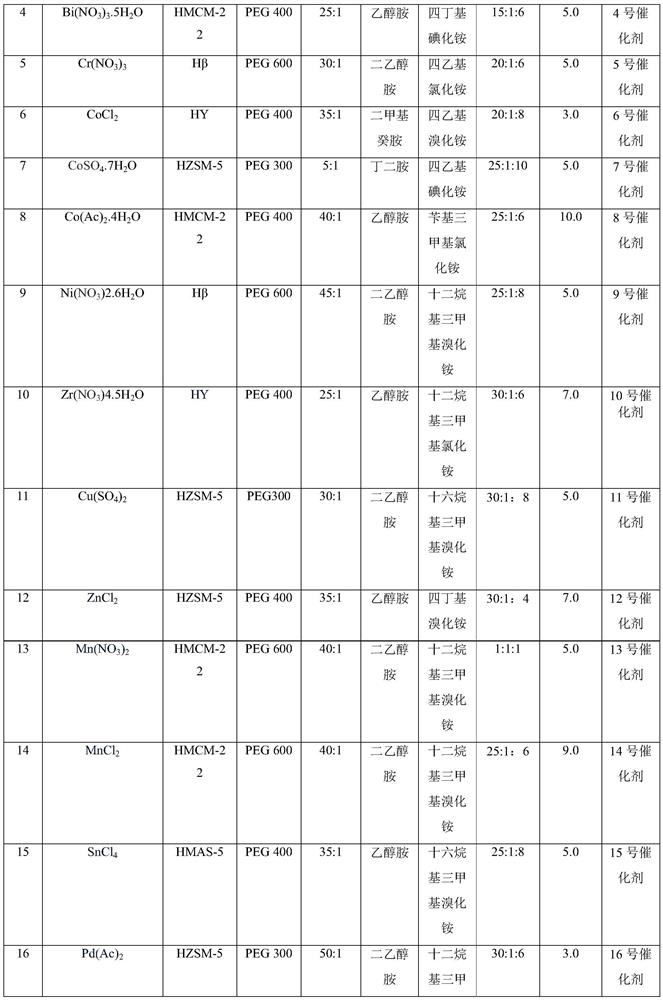
[0072] At room temperature, put 50 g of dried HMAS-5 molecular sieves into a three-necked flask, add 300 mL of toluene to swell for 12 hours, add 80 mL of thionyl chloride, react at 70 °C for 10 hours, filter with suction, wash, and vacuum-dry at room temperature until constant Heavy. The above swollen carrier was dispersed into 300mL of toluene, 1250g of PEG 400 was added, and after reflux reaction for 8 hours, it was suction filtered, washed, air-dried, and vacuum-dried to constant weight at room temperature. Then the above carrier was dispersed into 300mL of toluene, 95.39g of ethanolamine (25:1) was added, refluxed for 6 hours, filtered with suction, washed, and vacuum-dried at room temperature to constant weight. Swell with 300mL of toluene and ethanol mixed solvent (volume ratio 1:1) for 10 hours, add 10.14g of ferric chloride, 132.0g of dodecyltrimethylammonium chloride (MW.263.89), reflux for 24 hours, and filter with suction , washed, dried in the air, and vacuum-dri...
Embodiment 21-80
[0079] Embodiment 21-80 catalyst performance test embodiment
[0080] Catalyst performance testing is carried out as follows:
[0081]Mix prenyl aldehyde and prenol in a liquid storage tank according to a certain molar ratio (1:2.0), and when the temperature of the catalyst bed in the reactor reaches 70-120°C, it will continuously react in the fixed bed through an orifice flowmeter The feed rate is 1.67-16.7mL / min, and the residence time is 0.1-1.0 hours. The reaction occurs in the catalyst bed, and the unreacted prenol takes water out of the fixed bed and is separated by the oil-water separator. , the unreacted prenyl alcohol enters the liquid storage tank, and the water produced by the reaction is discharged as waste water; the product is discharged from the bottom of the reactor, and after the production liquid is analyzed by gas chromatography, 3-methyl-2-butene-1- The content of the aldehyde di-isopentenyl acetal is more than 98%, and it enters the cracking tower for cra...
Embodiment 81
[0097] Embodiment 81 Catalyst applies mechanically
[0098] The No. 1 catalyst mentioned above was selected for use, and the experiment of catalyst application was carried out. Soak the catalyst used in Example 21, Example 41, and Example 61 in a mixed solvent of toluene and ethanol (volume ratio 1:1) at 80°C, 100°C, and 120°C under stirring conditions for 10 hours, suction filter, and wash , air-dried, and vacuum-dried at room temperature to constant weight. The specific operation is the same as that in the catalyst performance test. The specific experimental data are shown in Table 5.
[0099] Table 5.1 Data of No. 1 Catalyst Used in Aldol Condensation Reaction
[0100]
[0101]
[0102] Note: the aldol condensation reaction conditions are the same as in Example 21; the conversion, selectivity, and yield in the table are the same as the definition of conversion, selectivity, and yield in Example 21.
[0103] Table 5.2 The data applied mechanically in the thermal cr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com