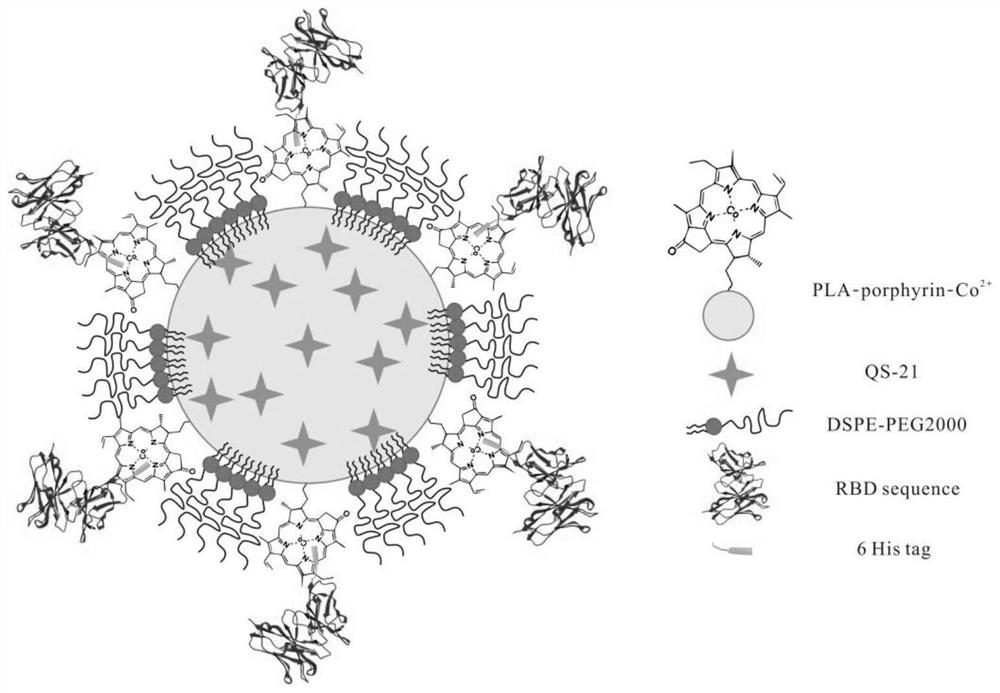
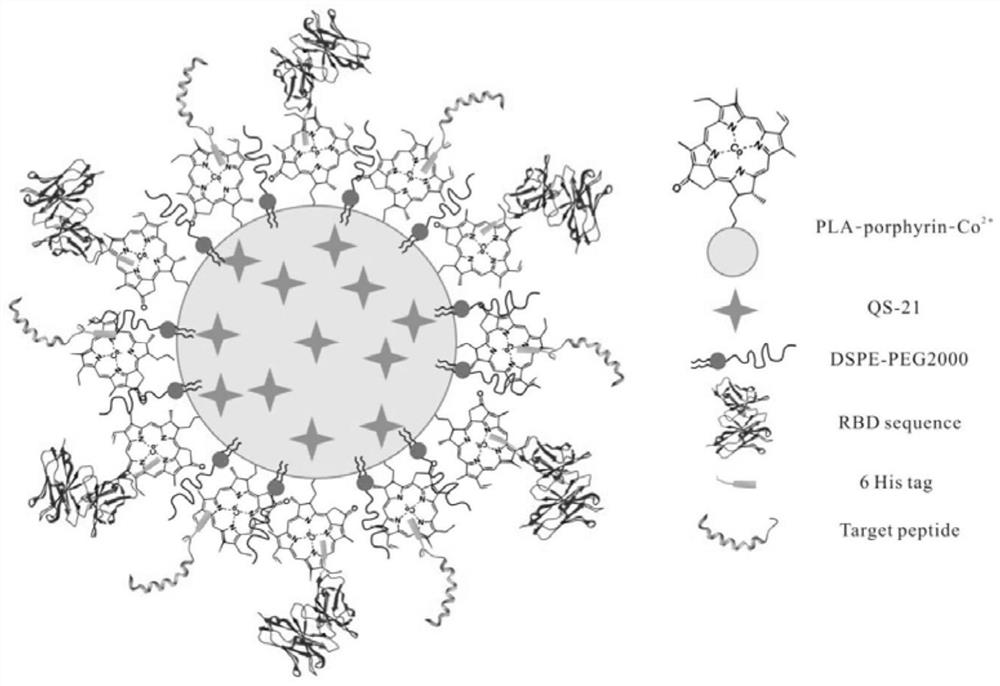
Novel coronavirus subprotein nano vaccine and preparation method and application thereof
A coronavirus and nano-vaccine technology, applied in the field of biomedicine, can solve the problems of difficulty in loading the new coronavirus S protein, inability to effectively large capacity, low immune response, etc., to achieve the prevention of new coronavirus infection, a new and efficient way and The effect of selection and efficient loading of antigen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 novel coronavirus subprotein nano-vaccine
[0041] 1. Synthesis of polylactic acid PLA
[0042] With n-hexanol as the initiator, stannous octoate (Sn(Oct) 2 ) as a catalyst to synthesize PLA by the method of ring-opening polymerization of L-lactide (L-LA) and D-lactide (D-LA), and its synthetic route is as follows:
[0043] The specific operation steps are:
[0044] Weigh 0.2500g of n-hexanol in the glove box and place it in a dry ampoule, add 2.1180g each of L-LA and D-LA, and add 0.31mL of pre-configured Sn(Oct) with a concentration of 0.02g / mL 2 solution, and finally add 21.0mL of dry toluene. Polymerization at 130°C, N 2 React in the environment for 2 days. The solution was settled with 200 mL of glacial ether, and the obtained product was dried at room temperature for 24 h. Then the product was dissolved in N,N-dimethylformamide (DMF), dialyzed in DMF through a dialysis bag (MWCO=1000D) for 48 hours to remove a small amount o...
Embodiment 2
[0071] The effect verification of embodiment 2 nano vaccine NPQ-RBD and NPQ-RBD-AP
[0072] 1. Characterization of Nano Vaccines
[0073] Nano-vaccine NPQ-RBD and NPQ-RBD-AP were respectively prepared by the method described in Example 1, and the particle size was detected to be between 100-200nm, and the nanoparticles in this range can effectively penetrate lymphatic vessels and be drained to the lymph nodes.
[0074]In order to verify the stability of the nano-vaccine NPQ-RBD and NPQ-RBD-AP carrying RBD fragments, the amino acid fragments on the nano-NPQ-RBD were labeled with FITC molecules; the nano-particles were dialyzed in saline at 4°C, and the fractions were collected every day Solution, using a microplate reader to detect the relative FITC fluorescence intensity in the nanometer, and set the nanoparticle NP-RBD control group without adjuvant, and the group injected with only RBD antigen, the detection results are as follows Figure 4 shown.
[0075] The results sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com