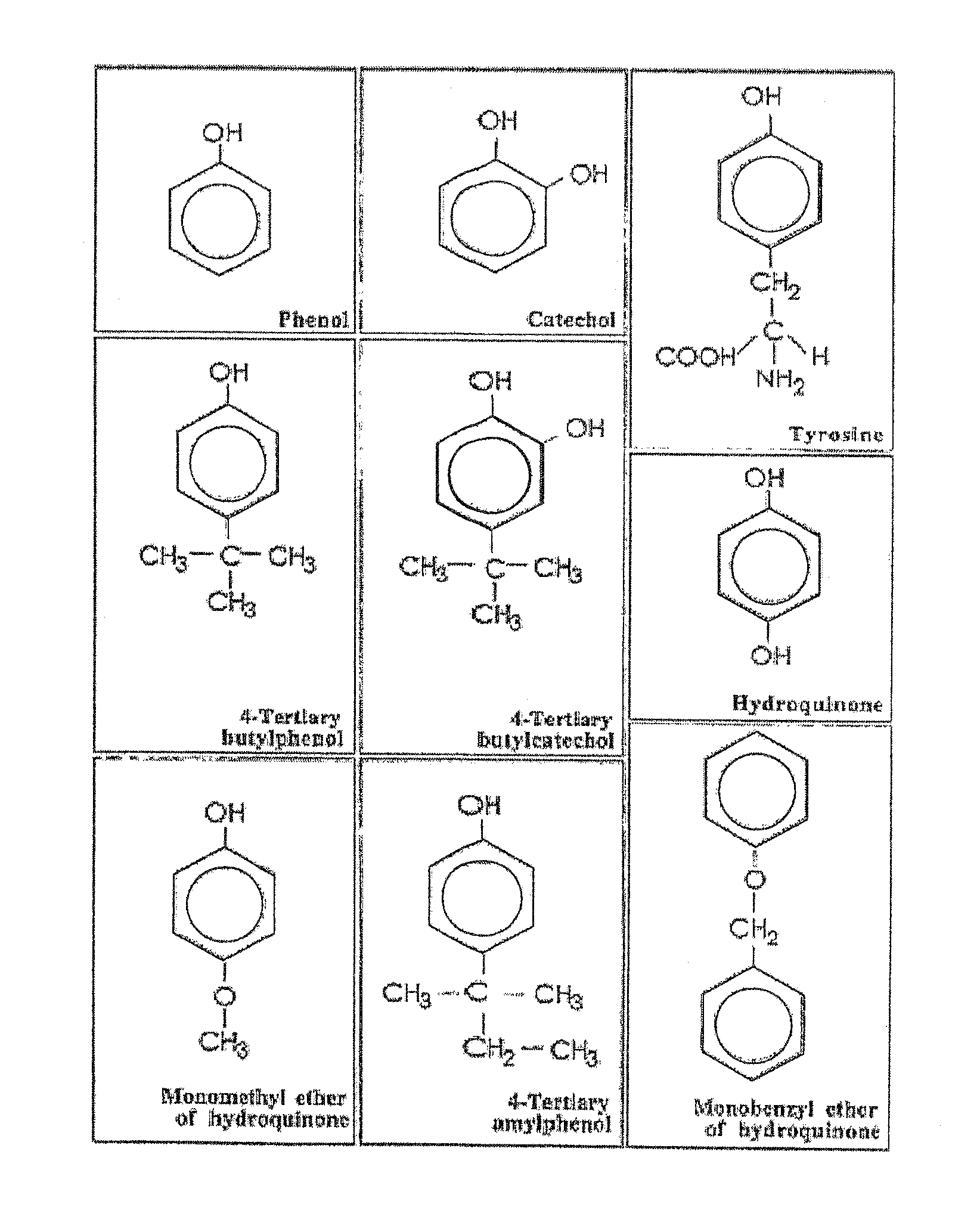
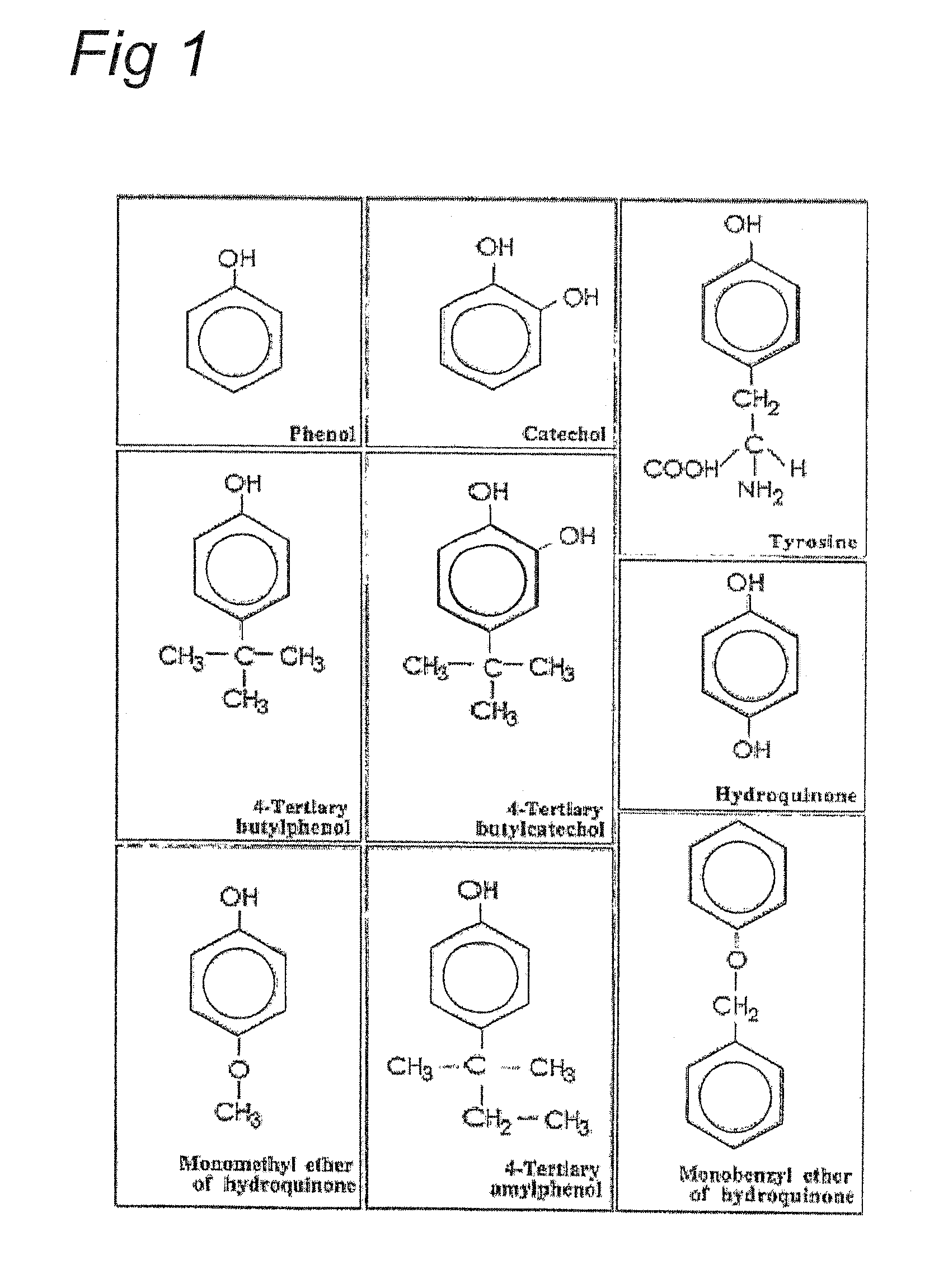
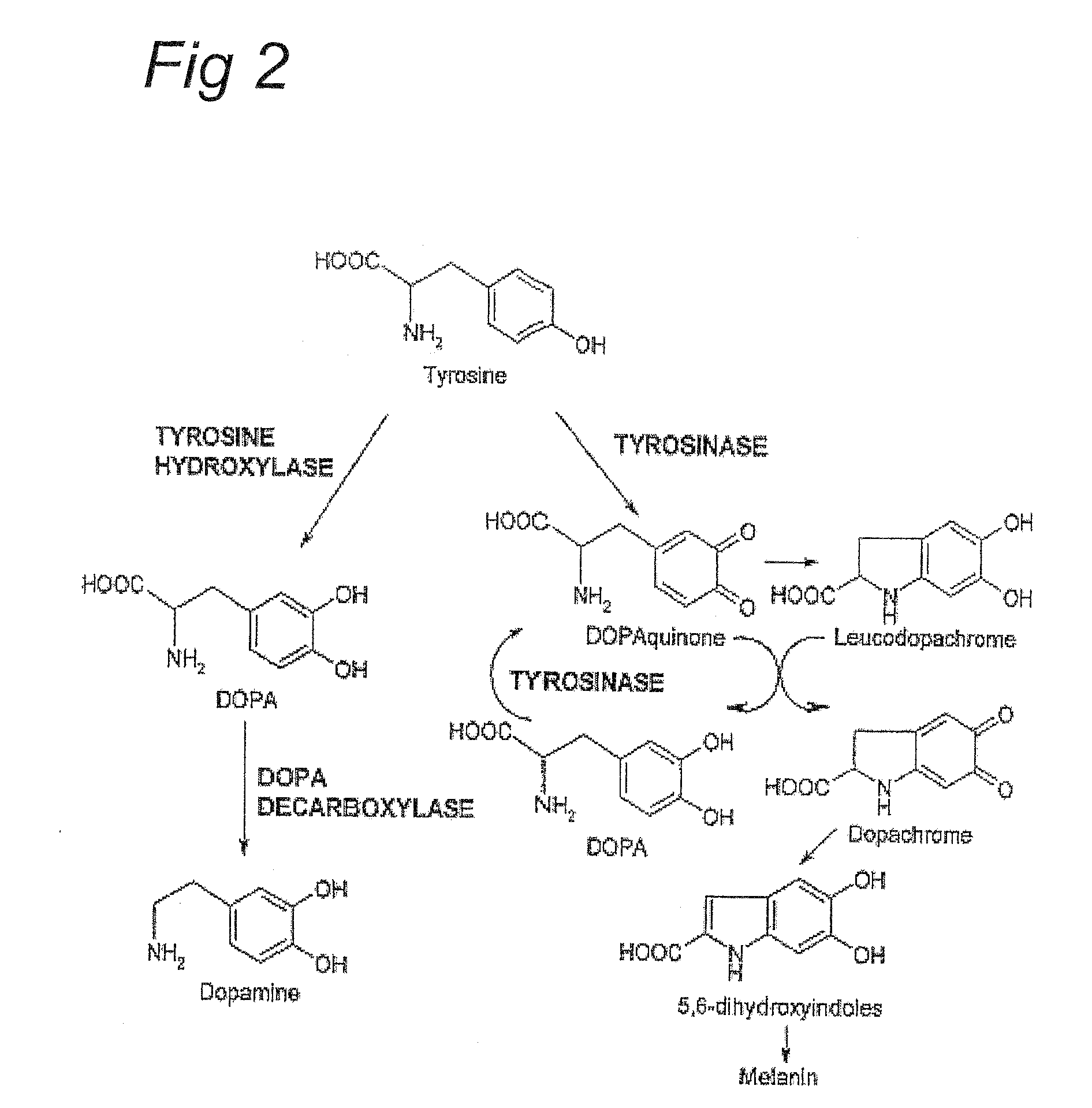
[0014]Contrary to the
high doses of tyrosinase surrogate substrates used in case of
intra arterial infusions as a
chemotherapy, a thousand fold lower systemic concentration is achieved in the current invention by local application of the active compounds on the lesions or injection in the melanoma
lesion, to evoke
sensitization of the
immune system against melanoma cells. The
intra arterial infusion of monophenols and benzenediols, in particular catechols, bypasses the melanoma
cell in the
skin or in the malignant
lesion. The invention comprises the use of ‘haptenized’ proteins, in particular proteins exhibiting a
tyrosinase activity and fragments of those proteins, which may be applied for eliciting immune responses
in vivo or
in vitro and for the manufacture of medicaments or vaccines. In particular embodiments of the invention, the reaction of the
immune system of a subject to be treated for
pigment cell malignancies such as melanoma, is further stimulated by
immune modulators applied on or in the
lesion, for instance compounds eliciting a local
inflammatory response. In a most preferred embodiment, the method and medicaments of the invention are applied in conjunction with steps to decrease the presence or the activity of regulatory T cells. Regulatory T cells function to prevent
autoimmunity and hamper attempts to elicit an immunogenic response against auto-antigens derived from melanocytes such as tyrosinase and tyrosinase related proteins TRP1 and TRP2. The invention provides different optional measures and steps to minimize the obstruction of regulatory T cells in the process of generating a cellular immunogenic response against the modified autoantigens of the invention.DETAILED DESCRIPTION
[0022]In case of the desired
sensitization of the
immune system of a
melanoma patient with a composition according to the invention, comprising monophenol or
benzenediol compounds, the T-
cell mediated cytotoxicity will be directed toward cells displaying the modified autoantigens, such as melanocytes, especially when the compound is applied topically on the lesion or injected intralesionally at relatively low doses. It will be particularly advantageous to repeat the administration to provide a
continuous exposure of modified
antigen to the immune
system and thereby boost the immune response. More preferably, a
slow release formulation of the
phenol or
catechol may be applied, providing a prolonged and sustained
exposure, while at the same time avoiding the
toxicity of
high peak doses of the compound to be used. During and
after treatment, all cells having a
melanin metabolism, including normal melanocytes in the
skin and hair, will disappear, locally and / or even systemically. This depigmenting effect is an unwanted but in the case of malignant and metastasizing melanoma an acceptable
side effect of the treatment.
[0027]In other embodiments two or more compounds of the group of monophenols and benzenediols may be used in combination, simultaneously in one composition or in separate compositions, simultaneously or sequentially applied to the lesion in situ. The use of several compounds has the
advantage that the auto-
antigen providing proteins that have
tyrosinase activity, will be modified with several compounds and / or reactive intermediates. Thereby several different ‘haptens’ on the tyrosinase enzymes will provide immune systems of treated subjects with a wider range of potential antigens that can be taken up and displayed by
HLA molecules. Since the ‘fit’ of an
antigen is among other factors highly dependent on HLA isotypes, this broadened approach will boost the potential immune response significantly. The mounting of a systemic auto-
immune reaction against all cells having tyrosinase activity provides an excellent means to combat also distant metastases, even micrometastases, that are not accessible to
surgical methods or radiotherapy and which are not accessible for
topical drug administration. The capability of melanomas to spread out and to form local and distant metastases is a common problem in treatment of patients suffering from malignant melanomas. This problem can be effectively eliminated with the methods and medicaments of this invention.
[0031]A pharmaceutically acceptable composition according to the invention comprises at least one monophenol or
benzenediol compound that can function as a substrate analogue of
tyrosine and is capable of reacting with proteins exhibiting tyrosinase activity; tyrosinase proteins or tyrosinase related proteins 1 and 2, or which can be activated by these enzymes into a
reactive intermediate which can subsequently react with tyrosinase or other related proteins. Optionally the composition may comprise one or more compounds selected from immune modifying compounds, immunogenic adjuvants and pharmaceutical excipients. Pharmaceutical excipients may comprise any
excipient known and customary in the art and for instance described in Remington; The Science and Practice of
Pharmacy, 21nd Edition 2005, University of Sciences in Philadelphia. Pharmaceutical compositions and medicaments of the invention may thus comprise binders such as
lactose,
cellulose and derivatives thereof,
polyvinylpyrrolidone (PVP), humectants, disintegration promoters, lubricants, disintegrants,
starch and derivatives thereof,
sugar solubilizers, anti-oxidants, preservatives, immuno-stimulatory adjuvants or other excipients. The invention provides methods and means to formulate and manufacture new medicaments and / or pharmaceutical formulations for the treatment of melanomas by topical administration to sensitize the immune
system against
melanoma antigens. The composition is preferably a composition that is optimized for trans-epidermal delivery, and may comprise
skin penetrants or permeators and skin-
permeation enhancers such as organic solvents such as DMSO,
ethanol, or
propylene glycol, whereby the resulting medium (skin /
solvent) may have an increased
partition coefficient for the therapeutic compound(s). In another embodiment the composition is a so called
slow release formulation, which are known per se in the art of
pharmacy, and may for instance comprise a release controlling
polymer, -gel or -matrix, forming a depository of the
active compound(s) (i.e. the monophenols and / or benzenediols), optionally surrounded or coated with a release controlling
coating or membrane or
biodegradable polymer, providing a slow but continued administration and / or release of the active compounds. Topical delivery compositions comprise ointments, pastes, gels, medicated powders, creams, lotions, aerosols, sprays, foams and medicated adhesives. Medicated adhesives, such as depositories on patches, allow a
sustained delivery of the
drug over days in many cases at a
constant rate. Alternatively, the composition may also comprise a pharmaceutically acceptable liquid formulation which may be injected directly into the lesion.
 Login to View More
Login to View More