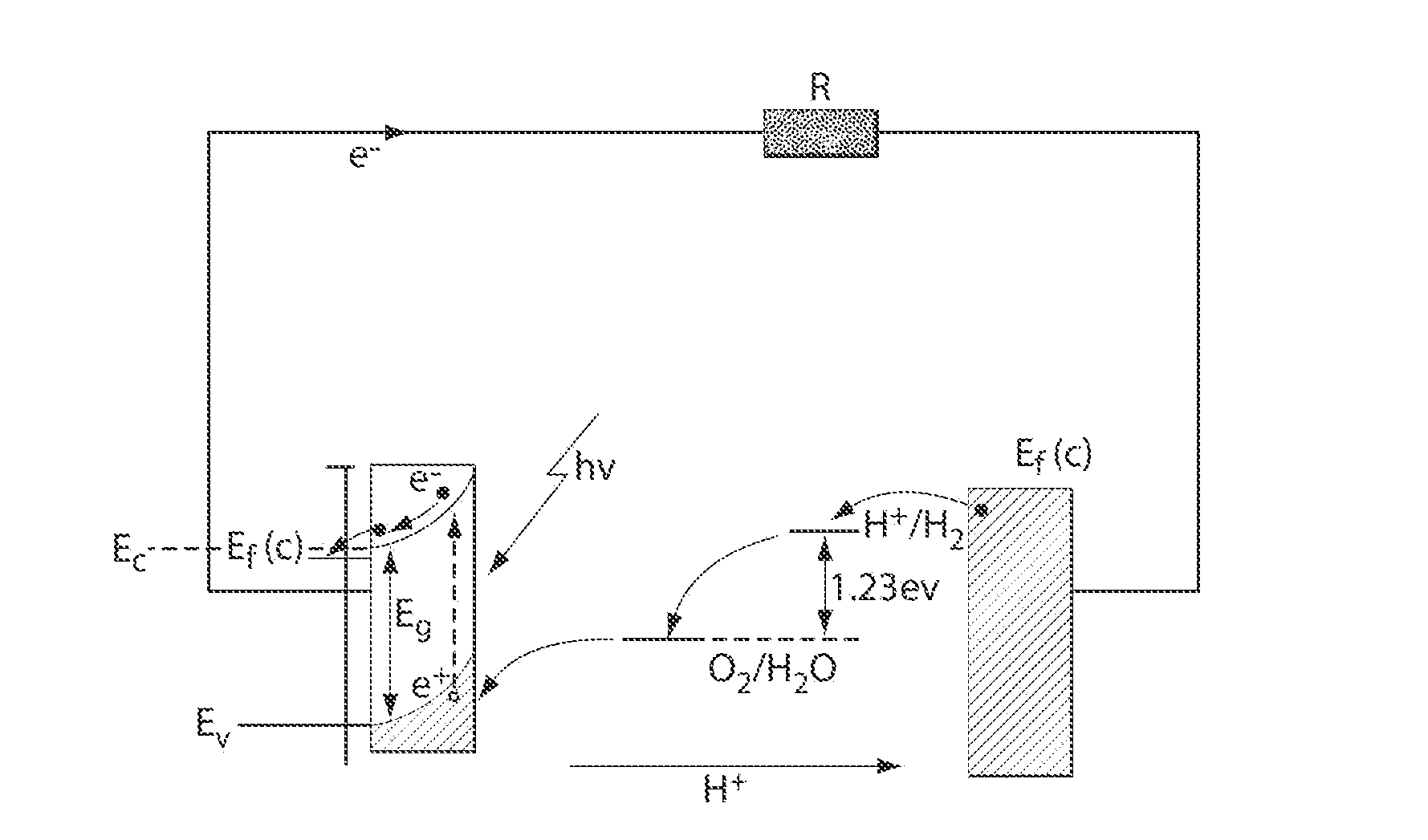
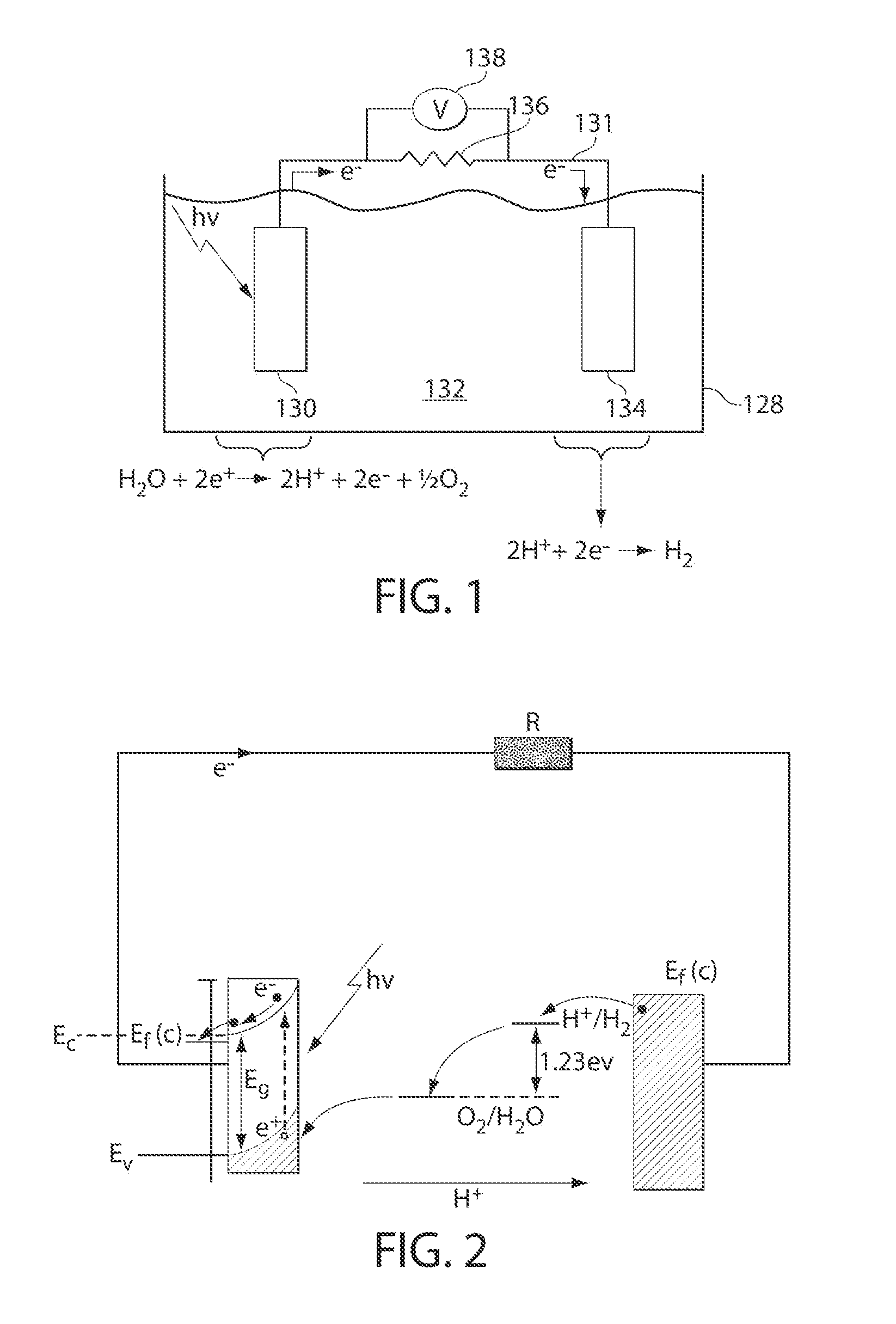
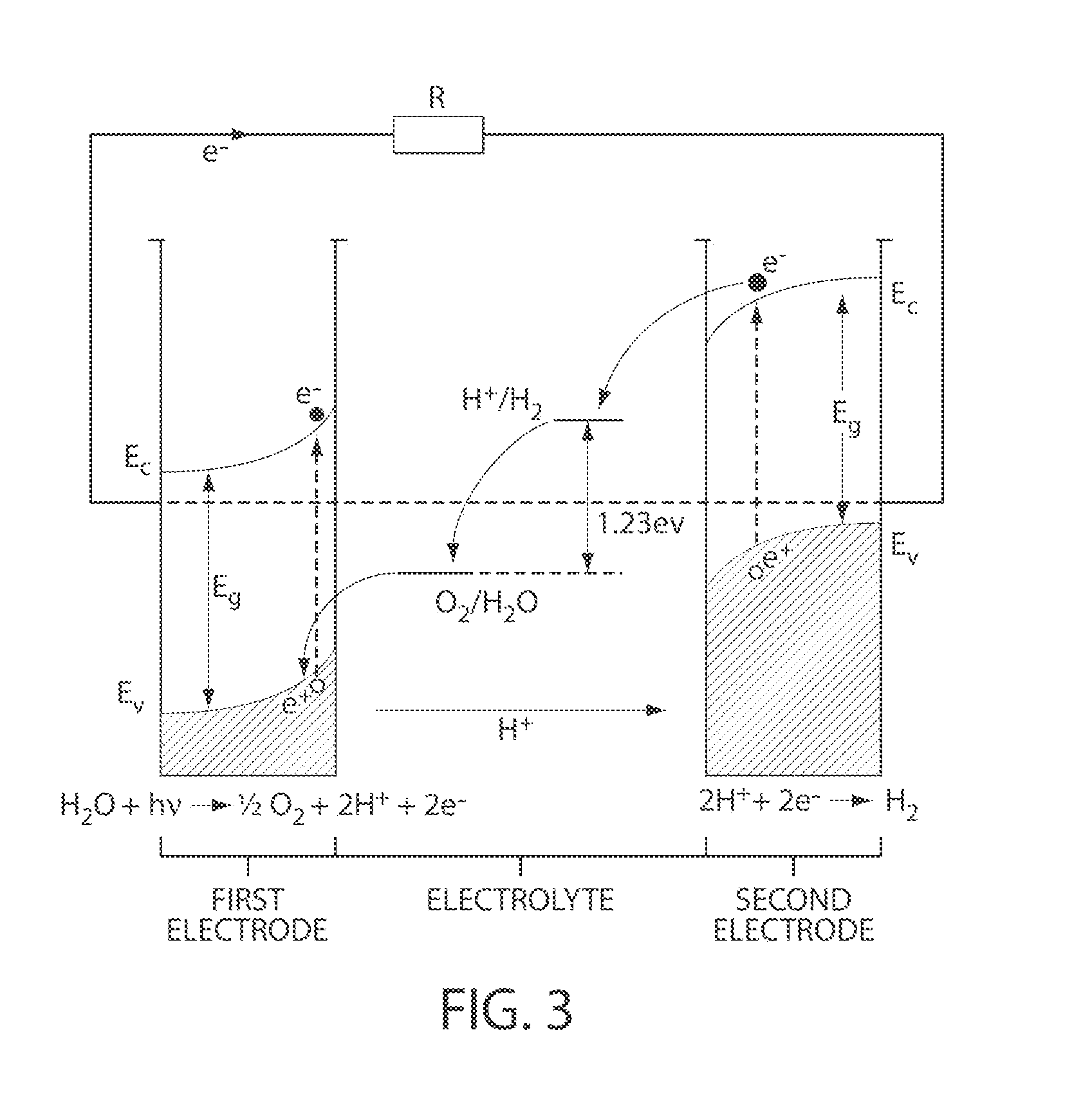
Catalytic materials, photoanodes, and photoelectrochemical cells for water electrolysis and other, electrochemical techniques
a photoelectrochemical and photoanode technology, applied in the field of photoanodes, can solve the problems of limiting the energy conversion efficiency, the greatest challenge of water electrolysis, and the general composition of expensive materials of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0209]The following example describes non-limiting examples of methods for deposition of a catalytic material comprising cobalt (Co-OEC) onto a photoactive material (e.g., a semiconductor, CdS). The method comprises, in this embodiment, providing a solution comprising metal ionic species and anionic species, providing a photoactive electrode, and causing the metal ionic species and the anionic species to form a catalytic material associated with the photoactive electrode by application of a voltage (e.g., by an external power source or by exposure to a light source) to the photoactive electrode.
[0210]Materials. Cadmium sulfate, thiourea, ammonium acetate, ammonium hydroxide solution (28% NH3), cobalt nitrate, methylphosphonic acid (Aldrich) and fluorine-doped tin oxide (FTO) coated glass substrate (Solaronix) were used as received.
[0211]CdS Film Preparation. Thin films of CdS were prepared on FTO-coated glass substrates by the chemical bath deposition technique. An Erlenmeyer flask ...
example 2
[0214]The following prophetic example describes methods for formation of a Co-OEC functionalized photoanode and characterization of the enhanced photoassisted water oxidation reaction rate.
[0215]Nanostructured iron oxide semiconductor (α-Fe2O3) may be grown on electrically conductive FTO-coated glass substrates by the atmospheric chemical vapor deposition (CVD) technique as described previously (e.g., See Kay et al., J. Am. Chem. Soc, 2006, 128, 15714-15721). The substrate may then be attached to a potentiostat as the working electrode and immersed in a solution of 0.1 M KPi (pH 7) and 0.5 mM Co(NO3)2. The electrode may then be biased at 1.1 V vs. Ag / AgCl reference for the electrodeposition of the Co-OEC catalyst as described in Example 1 and as done previously on ITO electrodes (e.g., see Kanan et al., Science, 2008, 321, 1072). The resulting α-Fe2O3 / Co-OEC electrode may then serve as a photoanode.
[0216]The α-Fe2O3 / Co-OEC photoanode may exhibit an enhanced rate for photoassisted wa...
example 3
[0217]The following prophetic example describes non-limiting methods for water oxidation, O2 gas evolution, and detection using Co-OEC functionalized photoanodes.
[0218]A Co-OEC functionalized photoanode (e.g., as prepared according to Example 1 or 2, or otherwise as described herein) may be attached to a potentiostat and serves as the working electrode for this experiment. The working electrode may be immersed in a buffered aqueous solution (e.g., 1 M KPi, pH 7) along with a reference electrode (e.g., Ag / AgCl) and an auxiliary electrode (e.g., Pt wire). The entire experiment may then be sealed from the environment (e.g., using rubber septa in ground glass joints attached to the electrochemical cell housing) and purged of air by bubbling with He gas (or other inert gas, e.g., N2, Ar). The photoanode may then be biased at some potential relative to the reference electrode (e.g., 02. This may be confirmed by operation of the photoelectrochemical cell in water containing some fraction o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| voltage | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com