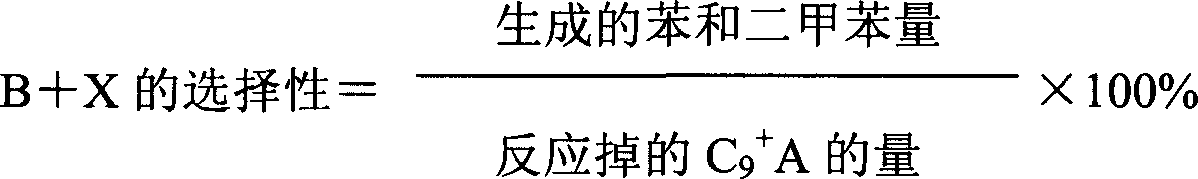Method used for heavy arene light formation and alkyl transfer
A technology for heavy aromatics and transalkylation, applied in chemical instruments and methods, hydrocarbon cracking to produce hydrocarbons, organic chemistry, etc. The effect of improving acid strength and acid content, and good technical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
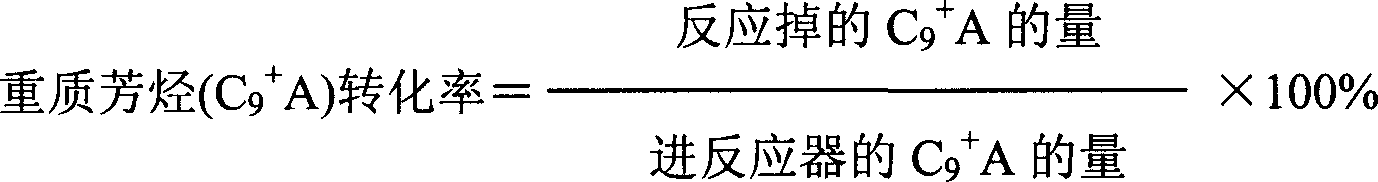
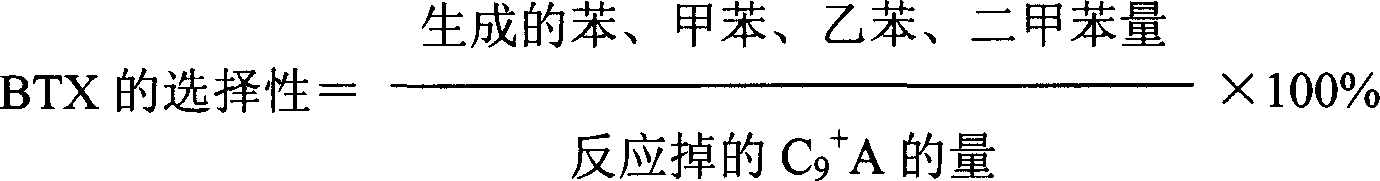
Method used
Image
Examples
Embodiment 1~5
[0032] Synthesis of BEA / MOR intergrowth molecular sieve used in the present invention
[0033] The BEA / MOR symbiotic molecular sieve used is synthesized according to Chinese patent ZL 02120815.8.
[0034] According to a certain proportion, sodium aluminate (analytically pure, commercially available), silica sol (industrial product, content 25% by weight), tetraethylammonium bromide (content 98% by weight, chemically pure), ammonia water (containing NH 3 25% by weight, analytically pure, commercially available), tetraethylammonium hydroxide (content 25% by weight, commercially available), sodium fluoride (analytically pure, commercially available), sodium hydroxide (analytically pure, commercially available product) to form a reaction system, mix evenly, transfer to a stainless steel crystallization kettle, and crystallize at 140-160°C for 1-3 days. After the crystallization, suction filtration, washing, and drying were carried out to obtain BEA / MOR intergrowth molecular siev...
Embodiment 6~10
[0038] Take the five different BEA / MOR symbiotic molecular sieves prepared in Examples 1-5, and exchange them with 1.0 M ammonium nitrate solution at 90° C. for 4 hours at a constant temperature; after filtering, repeat 3 times, and wash the filter cake with deionized water. The filter cake was dried at 110°C to obtain ammonium-type BEA / MOR symbiotic zeolite molecular sieves A1, B1, C1, D1, and E1.
[0039] A1, B1, C1, D1, E1 were respectively calcined at 550°C for 4 hours to prepare hydrogen-form BEA / MOR symbiotic zeolite molecular sieves A2, B2, C2, D2, E2.
[0040] Take 77.8 grams each of A2, B2, C2, D2, and E2, and combine them with Na 2 42.9 grams of pseudo-boehmite with an O content of less than 0.15% and a weight loss on ignition of 30% and an appropriate amount of extrusion aid scallop powder are mixed evenly, and 2 milliliters of chemically pure nitric acid and 60 milliliters of deionized water are added, fully mixed, kneaded, and carried out The catalysts A, B, C, D...
Embodiment 11
[0042] Take the BEA / MOR symbiotic molecular sieve prepared in Example 1, and exchange it with 1.0 M ammonium chloride solution at 90° C. for 2 hours at a constant temperature; after filtering, repeat 3 times, and wash the filter cake with deionized water. The filter cake was dried at 110°C to obtain ammonium-type BEA / MOR co-generated zeolite molecular sieve F1.
[0043] Take 44.4 grams of F1 molecular sieve and γ-Al 2 o 3 85.7 grams and an appropriate amount of extrusion aid Selina powder are mixed uniformly, add 2 milliliters of chemically pure nitric acid, and 60 milliliters of deionized water, fully mix, knead evenly, carry out extruding molding, drying, pelletizing, roasting and activating, and catalyst F is obtained .
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com