Method for catalytic oxidation of nitric oxide with low-temperature plasma modified catalyst
A technology of low-temperature plasma and nitrogen oxides, applied in the direction of separation methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high activation temperature, high energy consumption, high cost, etc., and achieve good selectivity and operation Simple, High-Converting Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Mix citric acid and manganese acetate with a molar ratio of 0.67, dissolve with deionized water and stir for 4 hours according to the solid-to-liquid ratio of 0.33; hour, when the foamy solid appears in the flask, take it out, and dry thoroughly in a 100°C oven for 6 hours to obtain the precursor of the catalyst; Manganese-based oxide catalyst A was obtained by treating for 5 hours under the condition of 0.2A, tableting, sieving and pulverizing into 40-60 mesh particles. The plasma adopts a coaxial dielectric barrier reactor. A stainless steel electrode with a diameter of 10 mm is inserted in the center of a corundum tube with an outer diameter of 25 mm and a thickness of 2.5 mm. The outer wall of the corundum tube is wound with stainless steel mesh as a high-voltage electrode, and a stainless steel wall plate is used as a low-voltage electrode.
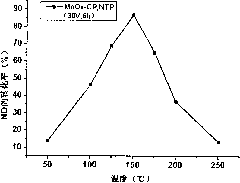
[0027] Catalytic oxidation reaction condition: Catalyst A 0.2g; Reactor inlet gas composition (volume ratio) NO 0.05%, O 2 ...
Embodiment 2
[0032] Mix citric acid and manganese acetate with a molar ratio of 0.50, dissolve with deionized water and stir for 4 hours according to the solid-to-liquid ratio of 0.25; after the stirring is completed, place it in a rotary evaporator for foaming treatment, and treat at 70°C 8 hours, when the foamy solid appears in the flask, take it out, dry it in an oven at 100°C for 6 hours, then bake it in air at 400°C for 6 hours, and finally place the catalyst in a low-temperature plasma. Treat under the condition of 1.0A for 6 hours, press into tablets, sieve and pulverize to make 40-60 mesh particles, and obtain manganese-based oxide catalyst B. The plasma is the same as in Example 1.
[0033] Catalytic oxidation reaction condition: Catalyst B 0.2g; Reactor inlet gas composition (volume ratio) NO 0.05%, O 2 3%, nitrogen as carrier gas; total gas flow rate 200cm 3 / min (airspeed=30600h -1 ).
[0034] Will contain NO, O 2 , N 2 The mixed gas is heated to 200°C through a glass tu...
Embodiment 3
[0038] Prepare the following three solutions: (1) dissolve 18.4g manganese acetate in 500ml deionized water; (2) dissolve 2.62g polyethylene glycol in 100ml deionized water; (3) dissolve 7.9g potassium permanganate In 300ml of deionized water, gradually add solution (2) to solution (1) while stirring, then add solution (3), start stirring at room temperature for 6 hours, filter the mixture, and collect the filter paper The solid matter on the surface was washed with deionized water for 3 to 4 times, and suction filtered; continued to dry in an oven at 100°C for 6 hours, and finally placed the prepared catalyst in a low-temperature plasma for treatment, with an input voltage of 30V and a current of 0.5 A, the processing time is 6h. Manganese-based oxide catalyst C is prepared by grinding, tableting and sieving to make catalyst particles of 40-60 mesh.
[0039] Catalytic oxidation reaction conditions: catalyst C 0.2g; Reactor inlet gas composition (volume ratio) NO 0.05%, O 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com