A method of transesterification to produce (meth)acryloyloxyethyltrimethylammonium chloride
A technology of acryloyloxyethyltrimethylammonium chloride and transesterification, which is applied in chemical instruments and methods, preparation of aminohydroxy compounds, preparation of organic compounds, etc. The effect of high rate and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The preparation of embodiment 1 acryloyloxyethyltrimethylammonium chloride
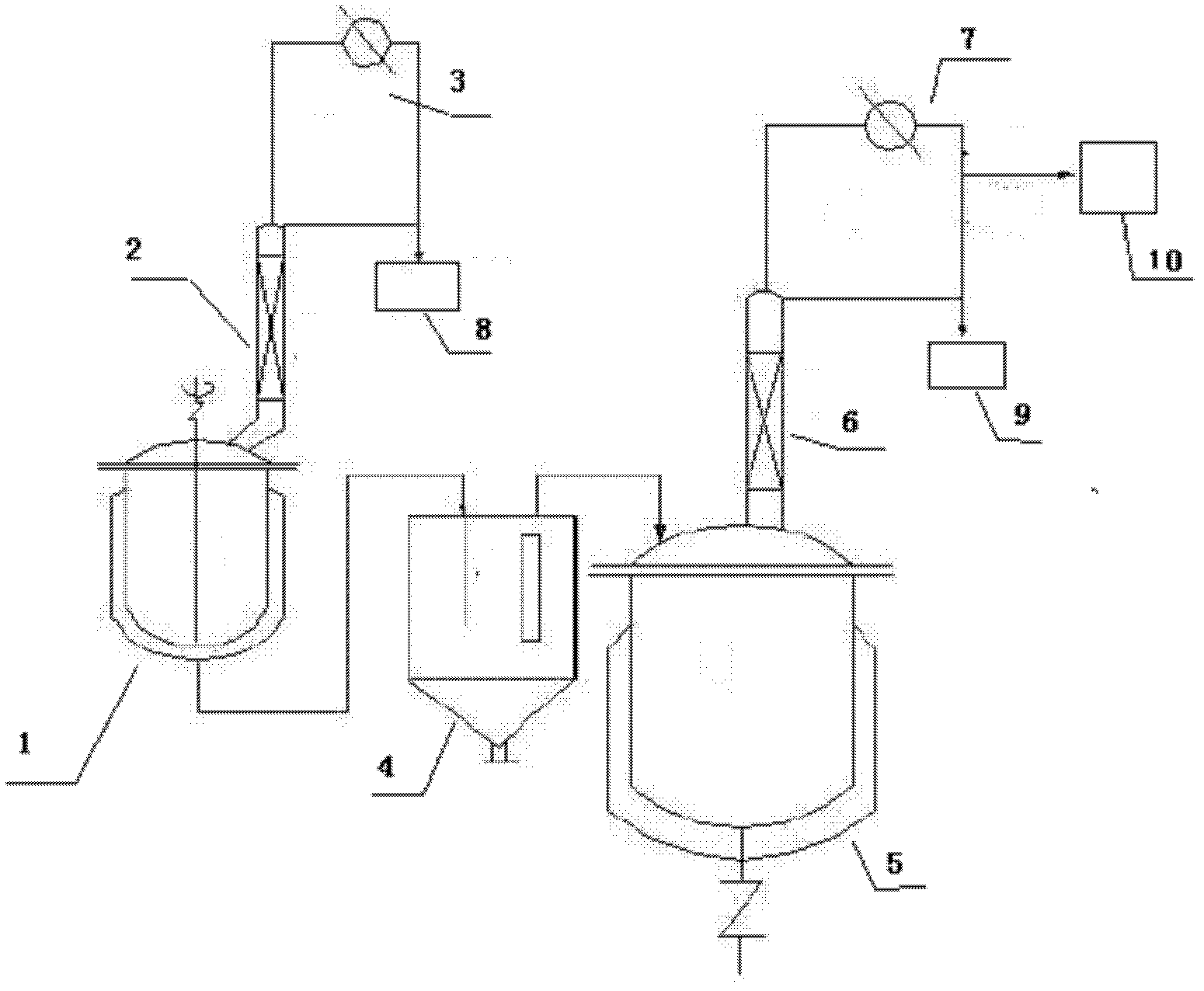
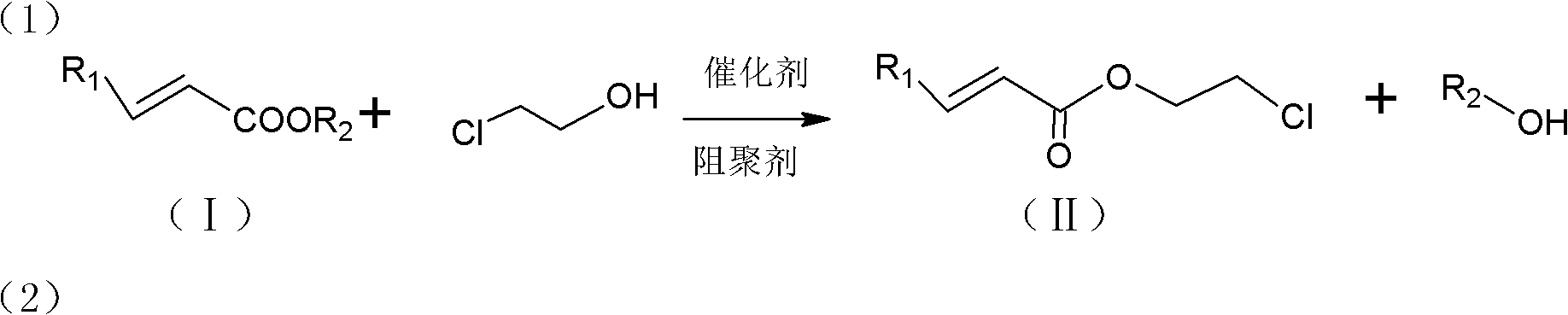
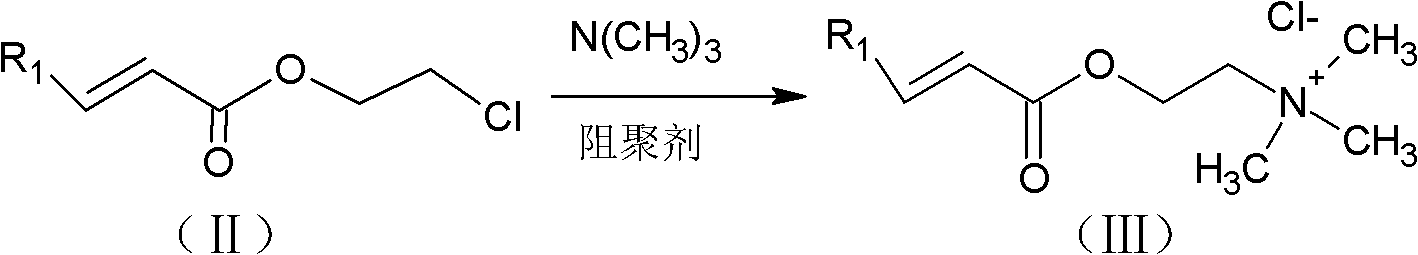
[0038] The first step, in the 3000 liters of reactors of packing distillation column that is equipped with mechanical stirrer, thermometer, condenser and theoretical plate number, add 1940 kilograms (24Kmol) methyl acrylate, 645 kilograms (8Kmol) 2- Chlorohydrin, 60 kilograms (0.48Kmol) of polymerization inhibitor p-hydroxyanisole, 128 kilograms (0.38Kmol) of catalyst tetra-n-butyl titanate, stirred and heated to reflux. Maintain the reaction temperature in the reactor at 90-130°C, the temperature at the top of the fractionation tower at 64-80°C, and the reflux ratio at 2-6, and simultaneously distill the methanol generated after transesterification out of the system. After reacting for 5-7 hours, stop the reaction. The material is transferred to the intermediate tank and settled. The upper layer liquid is transferred to the rectification kettle, and the residue in the reaction system is dist...
Embodiment 2-12
[0040] Embodiment 2-12: Same as embodiment 1, the difference is: ethyl acrylate, propyl acrylate, isopropyl acrylate, butyl acrylate, isobutyl acrylate, methyl methacrylate, ethyl methacrylate, methyl acrylate Propyl acrylate, isopropyl methacrylate, butyl methacrylate, and isobutyl methacrylate are used as raw materials in the first step reaction respectively.
[0041] The first step reaction, feed intake: 24Kmol (meth) acrylate, 645 kilograms (8Kmol) 2-chloroethanol, 60 kilograms (0.48Kmol) inhibitor p-hydroxyanisole, 128 kilograms (0.38Kmol) catalyst four titanate n-butyl ester.
[0042] Operation steps Repeat the first step reaction. The reaction temperature in the reactor is maintained at 90-130°C, the reflux ratio is 2-6, and the azeotrope of acrylate and small molecule alcohol is distilled out of the system at the same time. After reacting for 5-7 hours, stop the reaction. The material is transferred to the intermediate tank and settled. The upper liquid is transfer...
Embodiment 13-20
[0047] Embodiment 13-20: same as embodiment 1, the difference is that calcium oxide, aluminum oxide, zinc chloride, titanium isopropoxide, tetra-n-butyl titanate, aluminum isopropoxide, di-n-octyl tin oxide, p-toluene Sulfonic acid is used as a catalyst in the first step reaction respectively.
[0048] The first step reaction, feeding intake: 1940 kilograms (24Kmol) methyl acrylate, 645 kilograms (8Kmol) 2-chloroethanol, 60 kilograms (0.48Kmol) polymerization inhibitor p-hydroxyanisole, the catalyzer of 0.38Kmol.
[0049] Operation steps Repeat the first step reaction. Maintain the reaction temperature in the reactor at 110-120°C, the reflux ratio at 2-6, and distill the azeotrope of methyl acrylate and methanol out of the system at the same time. After reacting for 5-7 hours, stop the reaction. The material is transferred to the intermediate tank and settled. The upper layer liquid is transferred to the rectification kettle, and the residue in the reaction system is distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com