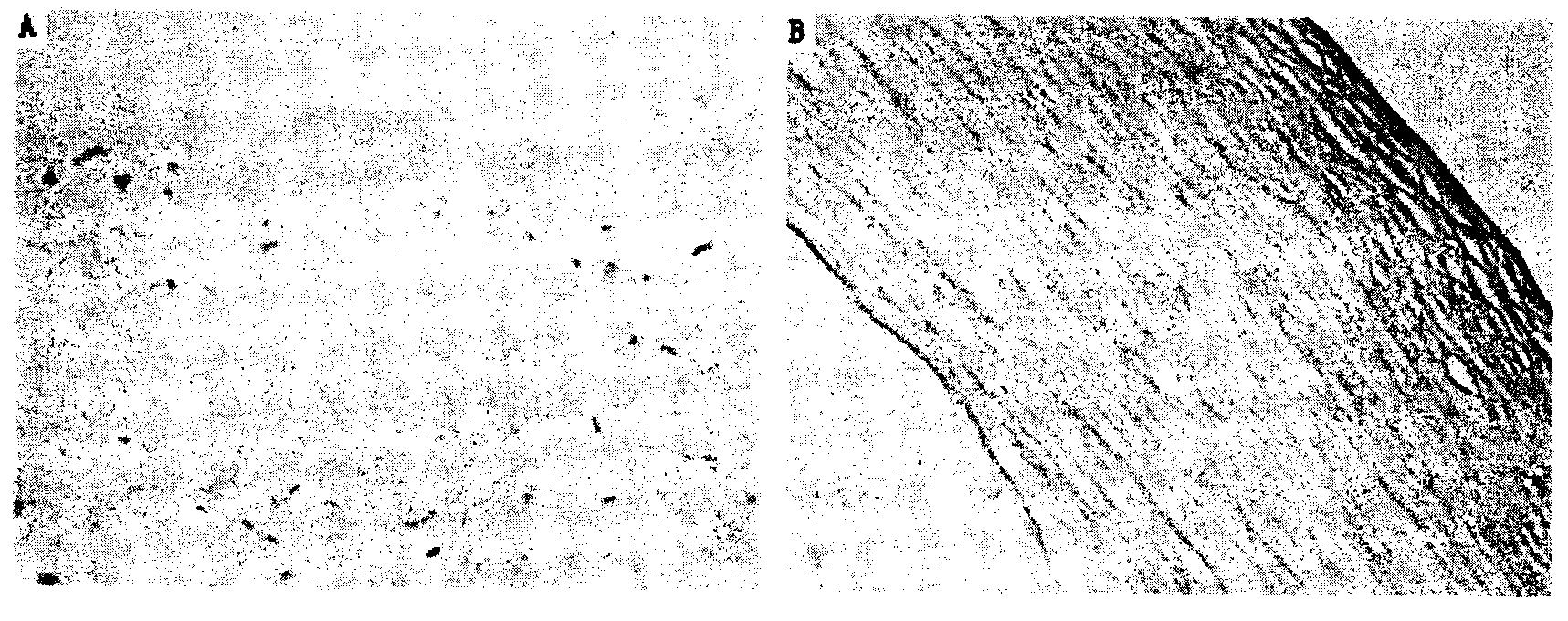
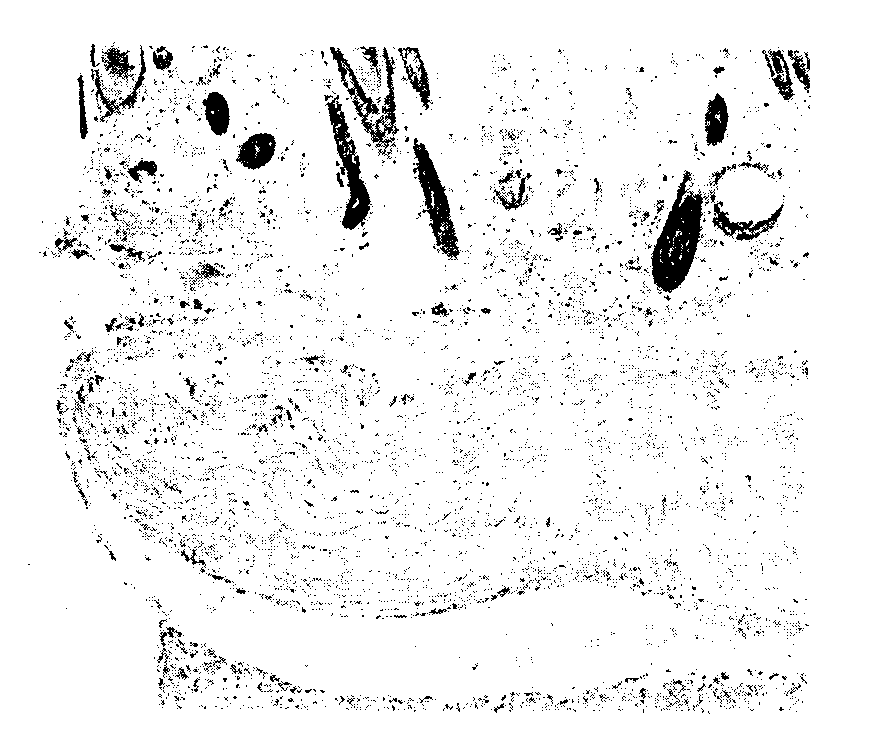
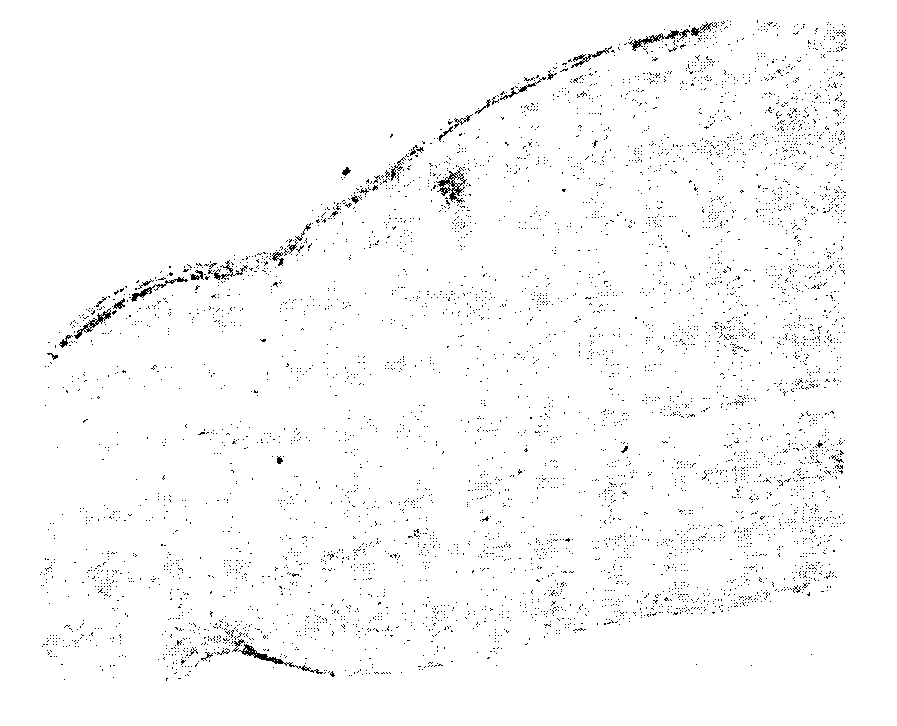
Method for preparing cornea lamina material
A corneal and lamellar technology, which is applied in the field of tissue engineering medical biomaterials, can solve the problems of disordered arrangement of lamellar structures between collagen molecules, reduced biological activity and fusion ability of materials, and low biological activity of corneal lamellar materials, so as to reduce pollution. risk, strong cell passaging ability, and controllable quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Treat the bovine eyeballs from the slaughterhouse, take the cornea-sclera complex and soak it in medical alcohol for 2 hours, then transfer it to 4°C normal saline for soaking, and set aside;
[0030] Step 1, decellularization treatment: put the cornea-sclera complex in 1mol / L sodium chloride aqueous solution containing 10ml / L Triton X-100, shake at 4°C for 5 hours; then place the cornea-sclera complex in In an aqueous solution containing 2ml / L of Triton X-100 and 0.1g / L of EDTA, shake at 4°C for 5 hours; repeat this step 6 times; hour; Obtain the decellularized cornea-sclera complex;
[0031] Step 2. Acquisition of amniotic epithelial stem cells: take primary amniotic epithelial stem cells and use primary cell culture medium to expand culture; the composition of primary cell culture medium is contained in commercial high-glucose DMEM culture medium, and EGF is 10ng / ml , human insulin 10mg / L, fetal bovine serum 150ml / L, L-glutamine 2×10 -3 mol / L, non-essential amino a...
Embodiment 2
[0038] Pig eyeballs from slaughterhouses were processed, and the cornea-sclera complex was soaked in medical alcohol for 2 hours, then soaked in 4°C normal saline, and set aside;
[0039] Step 1, decellularization treatment: put the cornea-sclera complex in 1mol / L sodium chloride aqueous solution containing 50ml / L Triton X-100, shake at 4°C for 5 hours; then place the cornea-sclera complex in In an aqueous solution containing 2ml / L Triton X-100 and 0.1g / L EDTA, shake at 4°C for 5 hours; repeat this step 8 times; obtain the decellularized cornea-sclera complex;
[0040] Step 2. Culture of amniotic epithelial stem cells: Take primary amniotic epithelial stem cells for culture; when the cell confluence reaches above 60%, digest with PBS buffer containing 4g / L trypsin for 6 minutes and collect the cell suspension, 600g Centrifuge for 5 minutes to obtain the cells; resuspend in culture medium A, and dilute with 1×10 4 / cm 2 The density of inoculated into the culture bottle and cu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile load | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com