Thymol ester derivatives, its preparation method and application thereof
A technology of drugs and compounds, applied in the preparation of carboxylate esters, cyanide reaction preparations, organic compounds, etc., can solve the problems of fast metabolism, low drug efficacy, high toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
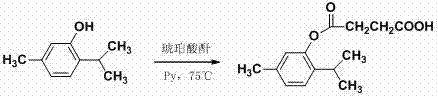
[0019] Preparation of thymol succinyl ester:
[0020]
[0021] Weigh thymol (0.52 g, 3.4 mmol) and succinic anhydride (0.92 g, 8.37 mmol) into a 100 mL three-necked flask equipped with a stirring device, add pyridine (20 mL), react at 75 ° C for 6 h, and detect the reaction by TLC [V (ethyl acetate): V (petroleum ether) = 4: 1 is the developer], after the reaction is basically completed, cool to room temperature. Remove pyridine by rotary evaporation under reduced pressure, add 20 mL of water, extract with ether 50 mL×3, combine the ether layers, wash with water (10 mL×3), dry with anhydrous sodium sulfate, recover ether under reduced pressure to obtain a yellow oily liquid, Add 50 mL of ether and 20 mL of ice water, let stand for 30 min, separate and collect the organic phase, extract the water phase twice with 20 ml of ether, combine the organic phases, add anhydrous Na 2 SO 4 (5 g) dried overnight. The organic solvent was recovered under reduced pressure to obtain a ...
Embodiment 2
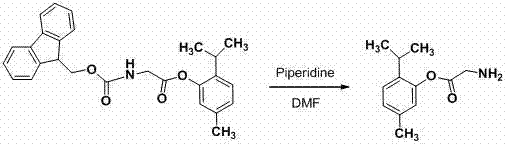
[0023] Preparation of Thymol Glycyl Ester
[0024]
[0025] In a 100 mL round-bottomed flask with a tail gas absorption device, add fluorenylmethoxycarbonyl glycine (4.9 g, 16.4 mmol), dichloromethane (30 mL), oxalyl chloride (15 mL). Heat to reflux and stir for 3 hours, evaporate under reduced pressure to remove the remaining oxalyl chloride to obtain fluorenylmethoxycarbonylglycyl chloride (F-moc-glycyl chloride) as a yellow solid.
[0026] Thymol (0.5 g, 3.3 mmol), pyridine (20.0 mL), and DMAP (0.05 g) were added to a 150 mL three-necked flask, and added dropwise at room temperature under stirring conditions to generate F-moc-glycyl chloride and toluene (20mL) mixed solution, after the dropwise addition, heated to 55°C, continued to react for 6 h, and monitored the reaction by TLC [developer: petroleum ether-ethyl acetate = 6:1, V / V]. After the basic reaction of the raw materials is complete, the toluene is distilled off under reduced pressure, the residue is dissolved ...
Embodiment 1 and Embodiment 2
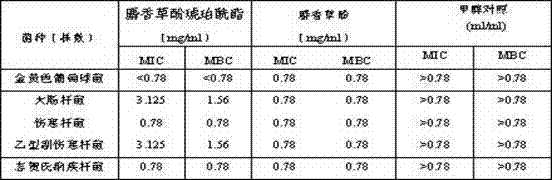
[0030] Embodiment 1 and embodiment 2 compound show good antibacterial activity in in vitro antibacterial experiment:
[0031] Strains: 1 strain of Staphylococcus aureus (standard strain ATCC6538), 1 strain of Escherichia coli (standard strain ATCC8099), 1 strain of typhoid bacillus, 1 strain of paratyphoid bacillus, 1 strain of Shigella dysenteriae and more than 5 strains. Medium: Nutrient agar (NA) lot number: 20101216; nutrient broth (NB) lot number: 20100806; product number: HB1019, manufacturer Qingdao Shangkeyuan Haibo Biological Co., Ltd. Preparation of test bacteria solution : Inoculate 5 kinds of bacterial strains into nutrient agar medium respectively. Cultivate at 37°C for 24 hours to select standard colonies and transplant them into liquid nutrient broth medium. After culturing at 37°C for 6 hours, adjust the bacterial concentration to 10 by McFarland Turbidimetry 8 cFu / ml for later use. Dissolution of the test drug: 2 solid compound monomers were dissolved s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com