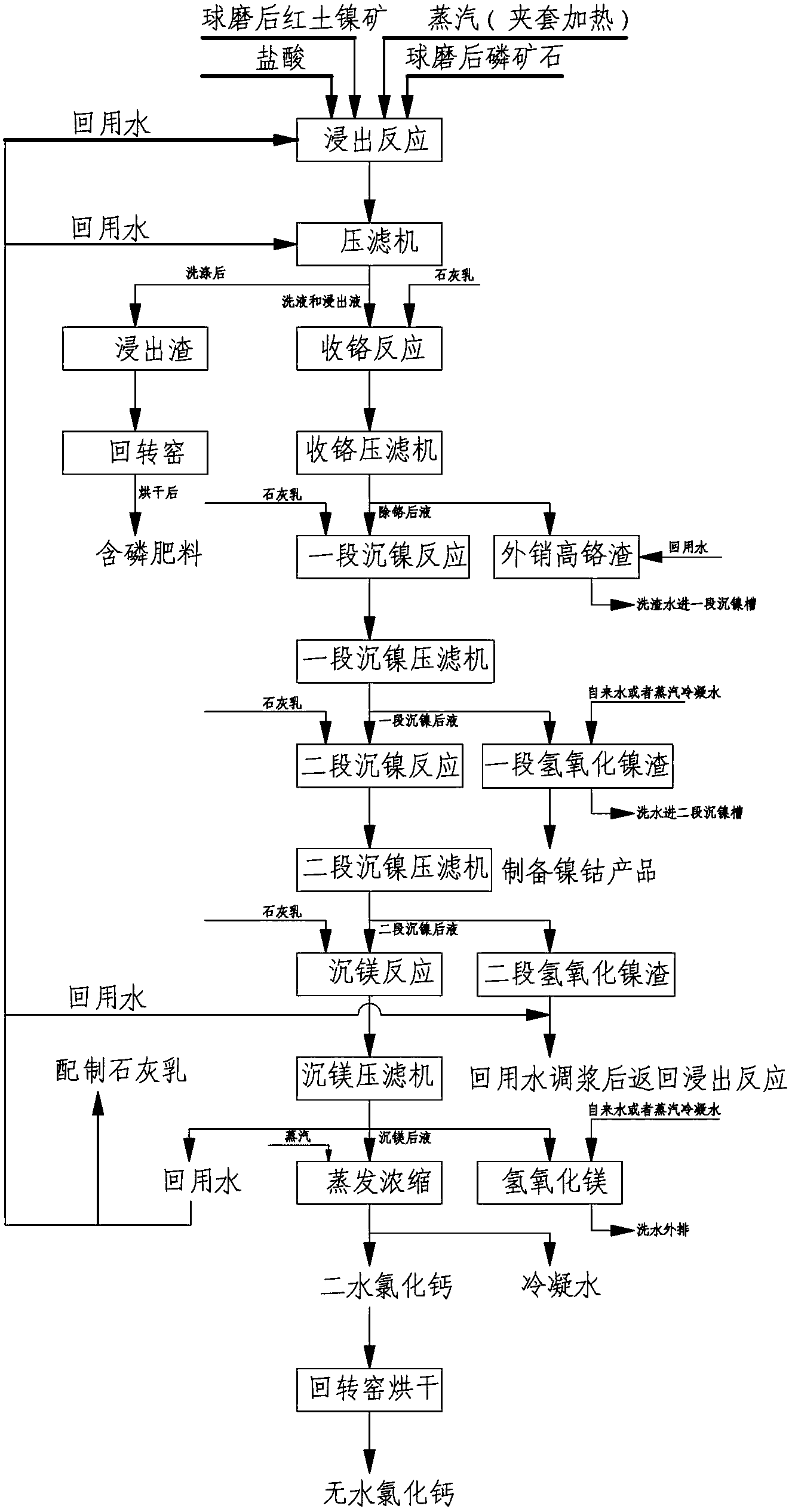
[0005] (1)
Atmospheric pressure sulfuric acid leaching method: This method uses
sulfuric acid as the leaching agent, controls certain liquid-
solid ratio, acidity, temperature and other
reaction conditions, and extracts nickel,
cobalt, iron,
magnesium and other metals from the ore under normal pressure. Leaching; then add neutralizer (limestone,
calcium hydroxide, or other alkaline substances) to remove impurities such as iron, aluminum,
silicon, etc., and obtain nickel
sulfate solution and
scrap slag after the
impurity-removing
slurry is press-filtered, and the
scrap slag is washed Outward
discharge, the nickel
sulfate solution uses
sodium hydroxide (or
calcium hydroxide,
magnesium hydroxide) for nickel
precipitation reaction; the obtained nickel
slag is acid-dissolved, extracted and
impurity-removed to obtain nickel-
cobalt deep-processed products; after nickel
precipitation, the liquid evaporates Recover magnesium sulfate by
crystallization, or directly add alkaline substances (limestone,
calcium hydroxide,
sodium carbonate, etc.) The
recovery rate of
metal and magnesium is low, the consumption of acid and alkali is high, and the amount of slag is large and cannot be comprehensively utilized, resulting in high production costs
[0006] (2) High-pressure sulfuric acid leaching method: This method uses sulfuric acid as the leaching agent, controls a certain
reaction temperature, and treats laterite ore under pressure; the pressure acid leaching process first began in Cuba in the 1950s. (MOA), since the 1990s, pressured acid leaching plants in Australia, such as Mullin-Mullin, Bloom, and Covas, have been put into operation one after another, but there have been many problems in terms of process and equipment. The basic process of this process is ore After crushing and pulping, enter the
autoclave for acid leaching at
high pressure (4-5MPa) and high temperature (230-260°C). After leaching, liquid-solid separation is carried out, and then the leaching liquid is neutralized and iron is removed. After
iron removal, the liquid is extracted.
Nickel-
cobalt separation can be further smelted to obtain different nickel-cobalt products according to different needs; the
recovery rate of nickel-cobalt in this process can reach more than 90%, but the
investment cost of this process is high, and the requirements for equipment and materials are relatively strict. Impurities have a great influence on the consumption of sulfuric acid, so this process is suitable for
processing laterite nickel ore containing less than 10% magnesium, especially less than 5%; in addition, during the operation of this process, equipment is prone to
fouling, which has a great
impact on production. The existence of a series of problems, and the high cost of equipment maintenance, and the inability to use slag reasonably, have affected the large-scale application and promotion of this method to a certain extent.
[0007] (3) Reduction
roasting-
ammonia leaching method: The reduction
roasting-
ammonia leaching process was invented by Professor Caron, so it is also called the Caron process, in which NH is used in the
ammonia leaching process 3 and CO 2 The nickel and cobalt in the roasted ore are converted into ammonia complexes and enter the solution. The
advantage of this process is that the reagents can be recycled and the consumption is small. The
disadvantage is that the
recovery rate of nickel and cobalt is low, and the recovery rate of nickel and cobalt is 75% and 60% respectively. left and right, and because the mineral materials need to be dried and roasted, the
energy consumption is relatively large, and the comprehensive recycling of resources cannot be realized
The alkali source used in the whole process is
sodium carbonate. Even if part of the magnesium
oxide is used as alkali in the process, the
alkalinity comes from
sodium carbonate, and the cost of alkali auxiliary materials is high; the process uses acid phosphoric acid Salt is used as a catalyst to participate in the reaction. Since acid phosphate is a chemical product, the purchase price is relatively high, resulting in high cost of production auxiliary materials; this process uses acid phosphate to remove iron and recover iron hydroxide and phosphate. This process adds new Due to the fact that
ferric hydroxide is a
colloid, the liquid-solid
separation process is relatively difficult, which will cause inconvenience in the production implementation process; this process carries out
evaporation and concentration recovery of sodium products, due to the addition of
sodium sulfate and other series products The value is extremely low, and the concentration in this
system is also low, and the recovery by
evaporation and concentration needs to be evaluated in terms of economy; in summary, there are still certain problems in the industrial application of this method
[0010] The choice of acid source and alkali source in the
hydrometallurgy process of laterite nickel ore: in the choice of acid source, sulfuric acid is mostly used as the acid source, and in the choice of alkali source, sodium alkaline salt (
sodium carbonate, hydroxide
Sodium, etc.),
Chinese Patent Application No. 201210202583.7 discloses a method for comprehensive recovery of valuable metals from laterite nickel ore. This method uses sulfuric acid as the acid source and milk of
lime as the alkali source. This acid-base source selection The
disadvantage is that calcium sulfate is more or less entrained in the products of using lime milk to collect
chromium, using lime milk to deposit nickel, and using lime milk to deposit magnesium. The adverse effects of the existence of this part of calcium sulfate are as follows: Aspects: one is that the purity of the product is reduced, and the other is that calcium sulfate dissolves into the
leachate during the
acid dissolution process, and the calcium ions in it will subsequently scale in the extraction and other sections, which will have a great
impact on the process
 Login to View More
Login to View More  Login to View More
Login to View More