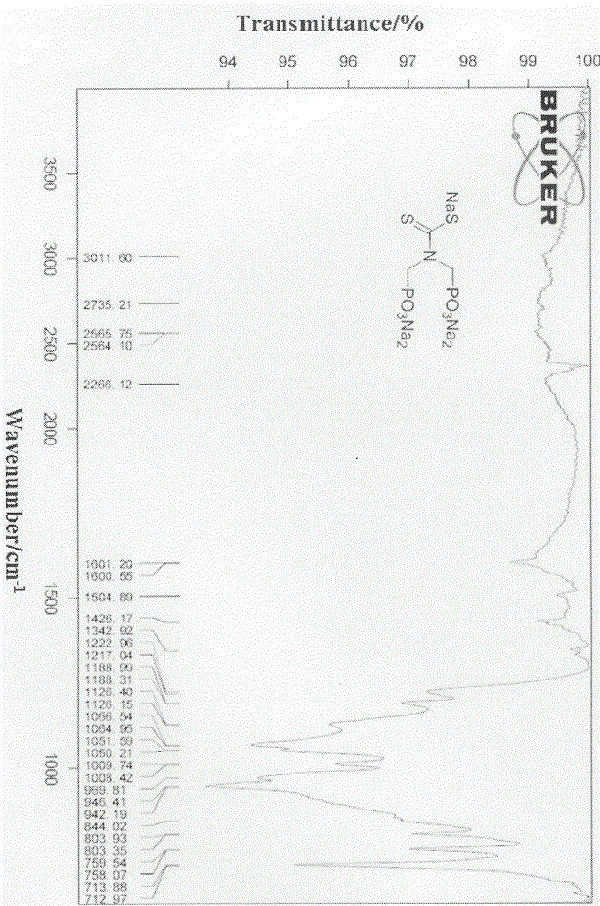
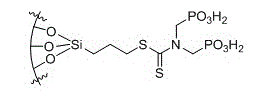
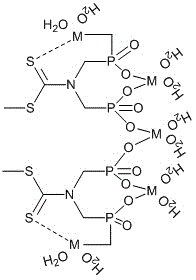
Silica gel absorbent for absorbing heavy metal ions and preparation method thereof
A silica gel adsorption and silica gel technology, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problems affecting the adsorption speed and efficiency, poor selective adsorption function, and still need to improve the adsorption effect, etc. Achieve the effects of enhanced adsorption rate and efficiency, stable treatment effect, and improved infiltration rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] 1. The preparation method of silica gel material is divided into functionalization of silica gel material, preparation of ligand thiocarbamate and preparation of silica gel adsorbent.
[0032] Functionalization of silica gel material: take chromatographic packing column silica gel (60-100 mesh, surface area 380 m 2 / g) in a round bottom flask, respectively in HNO 3 / H 2 Reflux in O (1:1) solution for 3 hours, HCl / H 2 O (1:1) solution was reacted at room temperature for 6 hours, and after the reaction, the solid product was obtained by suction filtration, which was washed with double distilled water and methanol in turn until neutral, and then obtained at 160 oC for 10 hours. Put the activated silica gel in a round bottom flask, add toluene, and raise the temperature to 80~140 o C, drop the silane coupling agent through the constant pressure dropping funnel. After the dropwise addition, at 80-140 o Continue to react for 12 to 24 hours under C; o C under vacuum dry...
experiment example 1
[0039] Experimental example 1: The application of silica gel adsorbent in the removal of heavy metal ions in electroplating wastewater.
[0040] Get 1000mL of electroplating plant discharge wastewater (a manufacturer in Zhuzhou, Hunan Province, sampling in January, 2013), add 50 grams of silica gel adsorbent prepared in the above method to it, at 25 o Oscillating in a constant temperature oscillator at C temperature to adsorption equilibrium, the filtrate was collected; the concentration of heavy metal ions before and after adsorption was measured by Optima5300 Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES).
[0041] Determination of heavy metal elements Before adsorption (mg / L) After adsorption (mg / L) Pb 77.146 0.008 Cr 4.526 not detected Cu 81.748 0.004 mn 7.813 not detected Cd 2.432 not detected Ni 40.934 0.002
[0042] Conclusion: The content of heavy metal ions in electroplating wastewater tr...
Embodiment 2
[0043] Example 2: The application of removing heavy metal ions in the extract of Eucommia bark.
[0044] Take 500 g of Eucommia bark (purchased in October 2012 in Cili County, Zhangjiajie, Hunan Province) commercially available, pulverize and sieve the powder into a 1L round bottom flask, add water until the traditional Chinese medicine is completely soaked in water, and soak for 30 minutes. Heat and boil for 45 minutes, filter and collect the filtrate; add distilled water to the filter cake again, heat and boil for 1 hour and then filter; combine the two filtrates, and distill under reduced pressure to obtain 54 g of a paste solid. Repeat the above operation to make three parts of the above paste solid; dissolve the paste solid in 200mL distilled water, add 5g of the prepared silica gel adsorbent respectively, oscillate in a constant temperature oscillator at room temperature to adsorption equilibrium, and collect the filtrate; use Optima5300 Inductively coupled plasma atomic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com