Diagnostic nano-drug which utilizes photothermal effect to assist penetration
A nano-medicine and photothermal technology, which is applied in the field of diagnostic and therapeutic nano-medicines and instant diagnostic and therapeutic nano-medicines, can solve the problems that the release kinetics cannot be completely consistent, it takes half an hour to several hours, and the space distance is different, so as to improve the curative effect, Increased blood flow and reduced systemic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079]
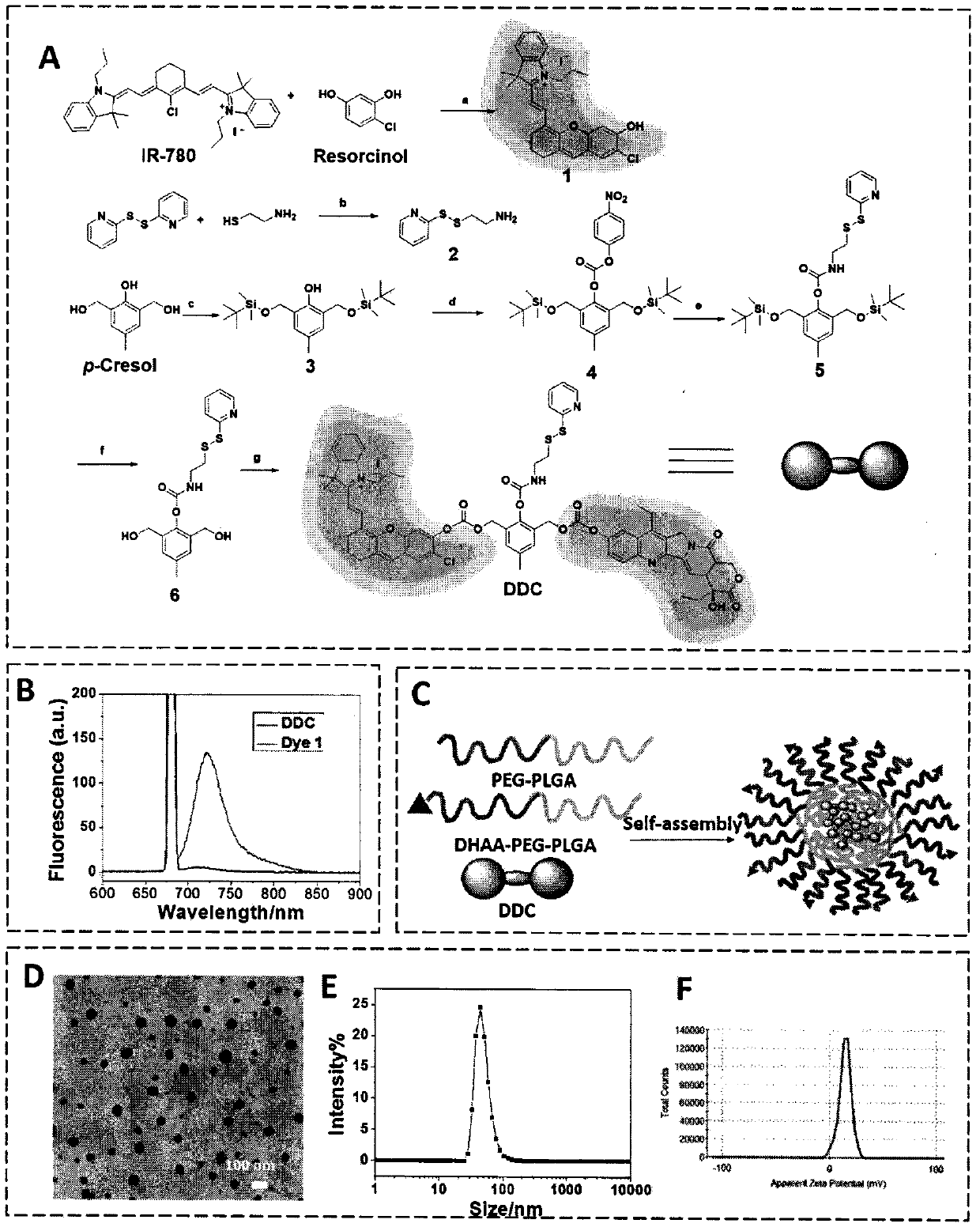
[0080] 4-Chlororesorcinol (140 mg, 0.95 mmol) and triethylamine (0.35 mL, 2.4 mmol) were dissolved in anhydrous DMF (1.5 mL) at room temperature under argon atmosphere and stirred for 30 minutes. IR-780 iodide (250 mg, 0.375 mmol) was dissolved in anhydrous DMF (1 mL) and slowly added to the above solution in the dark. The temperature was raised to 50°C and kept for 4 hours, then cooled to room temperature, pure water (10 mL) was added, and extracted with dichloromethane (20 mL×3). The organic phases were combined, dichloromethane was distilled off under reduced pressure, and the crude product was purified by preparative column chromatography (dichloromethane:ethanol=30:1, v:v) to obtain a pure product in the form of blue-green crystals (71 mg, 63%).
[0081] Rf=0.5 (dichloromethane:methanol=30:1, v:v); 1 H NMR (400MHz, CDCl 3 , δ, ppm): 8.13 (d, J=13.2Hz, 1H, H-4), 7.42 (s, 1H, H-3), 7.32-7.28 (m, 3H, H-1, 2and 27), 7.08 (t, J=7.6Hz, 1H, H-28), 6.86(d, J=4.0Hz,...
Embodiment 2
[0083]
[0084] At room temperature under nitrogen atmosphere, 2-aminoethanethiol (1.0g, 13mmol, 1eq) and 2,2'-dipyridyl disulfide (5.7g, 26mmol, 2eq) were dissolved in ethanol: acetic acid (20:1, v: v, 30 mL) in the mixed solvent and stirred overnight. The solvent was evaporated under reduced pressure, and the crude product was purified by preparative column chromatography (ethyl acetate:petroleum ether=1:1, v:v) to obtain a pure product in the form of a white wax (0.71 g, 29.4%).
[0085] Rf=0.3 (ethyl acetate: petroleum ether=1: 1, v: v); 1 H NMR (400MHz, CDCl 3 , δ, ppm): 8.42(dd, J1=4.4Hz, J2=1.6Hz, 1H, H-1), 7.63-7.54(m, 2H, H-2, 3), 7.08-7.03(m, 1H, H-4), 2.98-2.93(m, 2H, H-5, 6), 2.76(dt, J1=43.2Hz, J2=5.6Hz, 2H, H-7, 8), 1.88(s, w, 1H , H-9, 10); MS-ESI Calc.for C 7 h 11 N 2 S 2 [M+H] + 187.3, Found, 187.0.
Embodiment 3
[0087]
[0088] Imidazole (1.7g, 25mmol) and tert-butyldimethylsilyl chloride (TBSCl, 3.8g, 25mmol) were dissolved in dry DMF (10mL), 2-hydroxy-5-methyl-m-xylylenedimethanol (6g, 35.7mmol ) was dissolved in dry DMF (10 mL) and added to the above solution and stirred at room temperature for 2 hours, then diluted into diethyl ether (100 mL) and washed with pure water (100 mL×3). The organic phases were combined, diethyl ether was distilled off under reduced pressure, and the crude product was purified by preparative column chromatography (ethyl acetate:petroleum ether=5:95, v:v) to obtain a pure product in the form of a colorless oil (3.8g, 80% ).
[0089] Rf=0.8 (ethyl acetate: petroleum ether=1: 10, v: v); 1 H NMR (400MHz, CDCl 3 , δ, ppm): 8.02 (s, 1H, H-6), 6.90 (s, 2H, H-3), 4.82 (s, 4H, H-5), 2.26 (s, 3H, H-4), 0.96-0.89(m, 18H, H-1), 0.18-0.08(m, 12H, H-2); HRMS Calc.for C 21 h 40 NaO 3 Si 2 [M+Na] + 419.2408, Found, 419.2413.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com