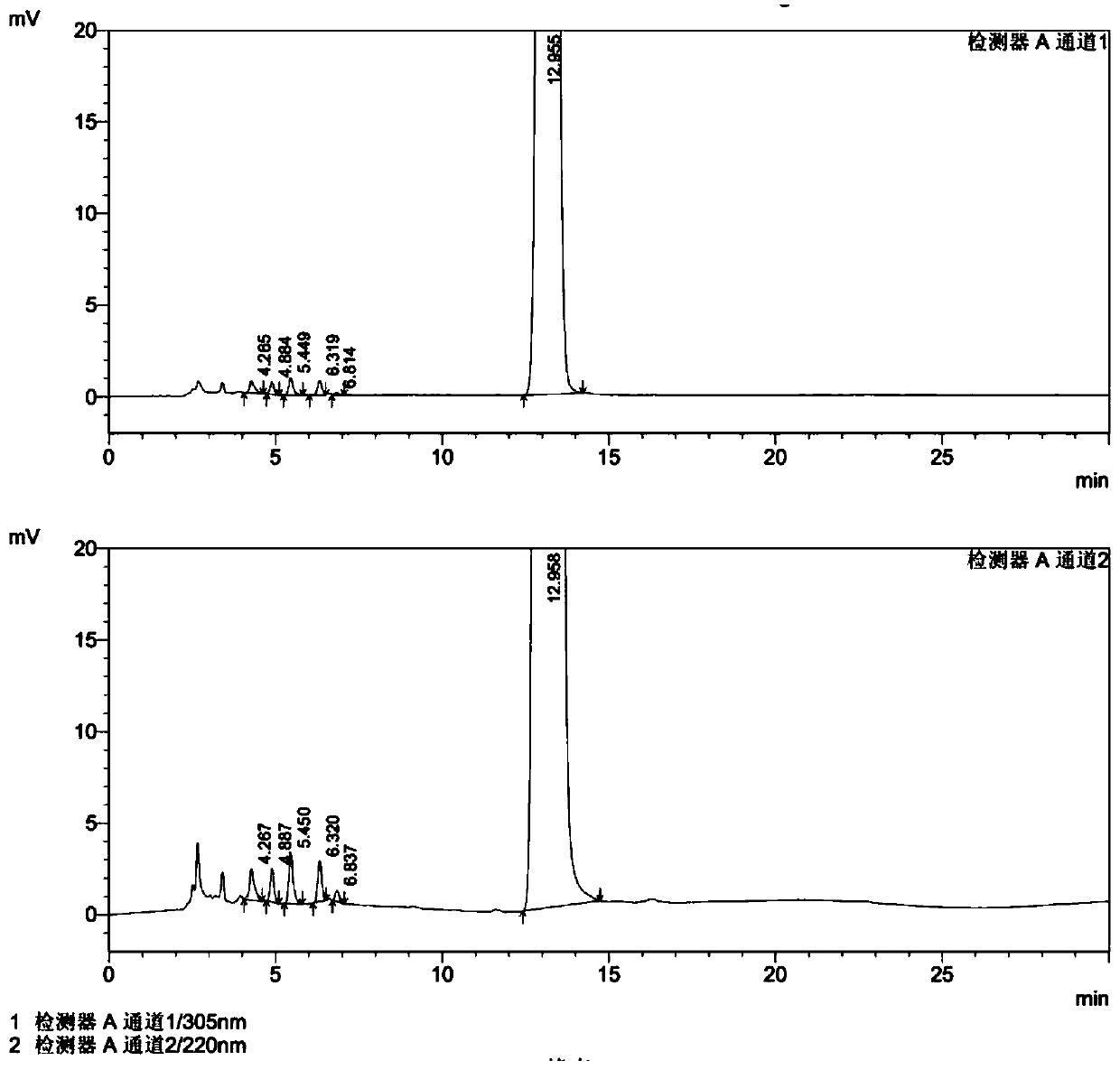
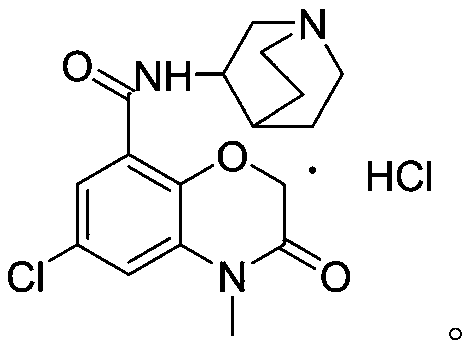
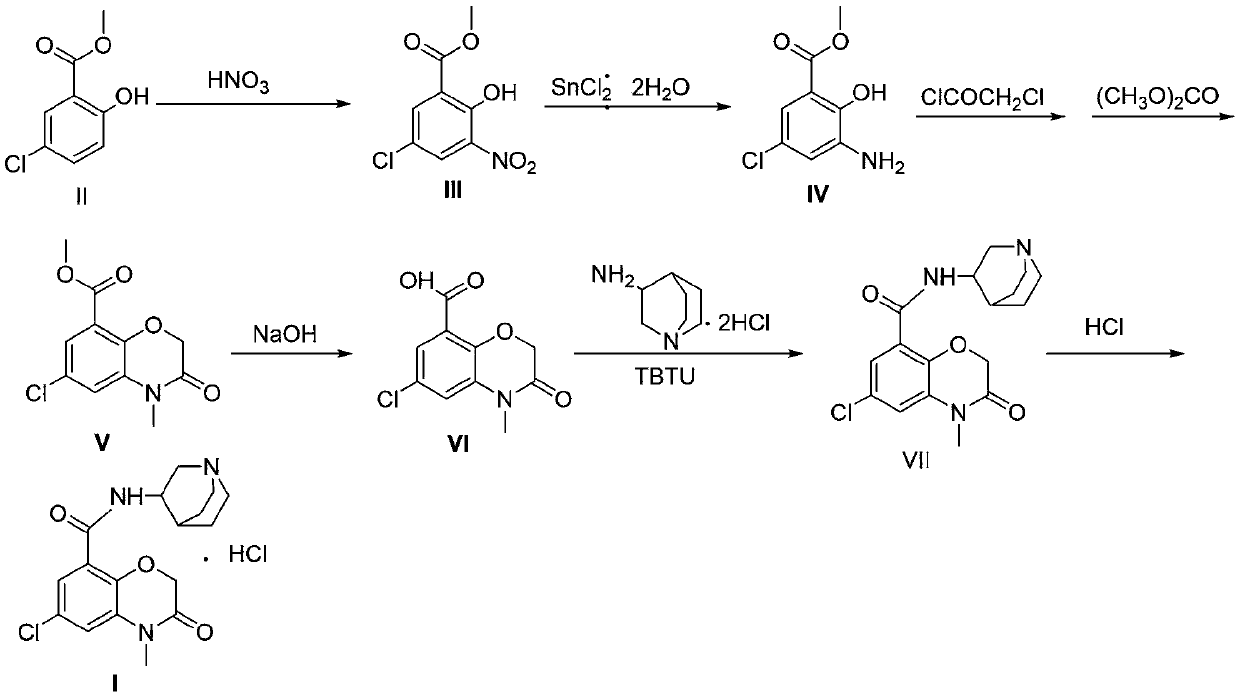
Novel preparation method of azasetron hydrochloride
A technology of azasetron hydrochloride and nitric acid, which is applied in the new field of preparation of azasetron hydrochloride, can solve the problems of complex post-processing, large environmental pollution, difficult refining, etc., and achieve industrial production, shorten the reaction route, and Handle simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation method of embodiment 1 compound III
[0044]Put 78.40g of methyl 5-chlorosalicylate into a 250mL dry three-necked bottle, add 120mL of acetic anhydride, cool to -5~0°C, add 19mL of fuming nitric acid dropwise under vigorous mechanical stirring, and react for 40min at the same temperature after dropping , poured into ice water (about 750 mL), filtered, washed with ice water and filtered to obtain a brick red solid, recrystallized from ethanol to obtain 87.82 g of light yellow powdery solid, yield 90.3%.
Embodiment 2
[0045] The preparation method of embodiment 2 compound IV
[0046] At room temperature, put 23.2g of compound III in a 250mL three-necked flask, add 100mL of ethyl acetate, stir until dissolved, then slowly add 70mL of concentrated hydrochloric acid dropwise, after the drop is complete, stir for 10min, add 35.5g of stannous chloride dihydrate in batches , Reaction at room temperature for 3.5h. Most of the solvent was removed by rotary evaporation, and the residue was poured into 200 mL of ice water, and adjusted to pH 7-8 with 2.5 mol / L sodium hydroxide solution. Extract with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 18.2 g of yellow-green solid IV, with a yield of 90%.
Embodiment 3
[0047] The preparation method of embodiment 3 compound V
[0048] Put 8.9 g of compound IV in a 500 mL three-necked flask, add 210 mL of dichloromethane, stir until completely dissolved, and then add 100 mL of saturated sodium bicarbonate solution dropwise. Cool to 0°C, add 6.6 g of chloroacetyl chloride dropwise under rapid stirring, and react at the same temperature for 3 hours after dropping. The organic layer was washed with water, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a reddish-brown solid. Add 55ml of DMF and 14.5g of potassium carbonate to the concentrate, stir until dissolved, and raise the temperature to 70°C for 2h. The temperature was raised to 75°C, and 6.3 g of dimethyl carbonate was added dropwise to the reaction liquid, and reacted for 3 hours. Pour into ice water, filter, wash the filter cake with water, and dry under reduced pressure to obtain white solid V10.38g, yield 92%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com