1-cyclohexylpyrazolone carboxylesterase 1 inhibitor as well as preparation and application thereof
A technology of cyclohexylpyrazolone carboxylate and cyclohexylpyrazolone, which is applied in the field of 1-cyclohexylpyrazolone carboxylate esterase inhibitors, can solve the problem of reducing drug concentration and affecting bioavailability To achieve the effect of improving drug activity, low cost and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
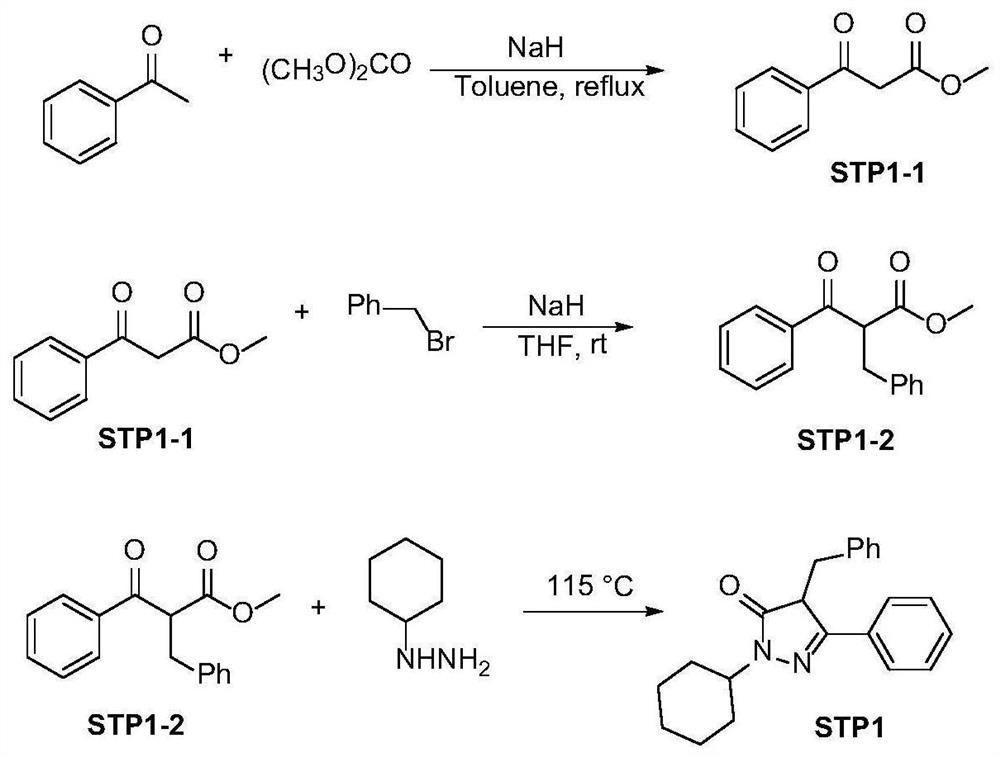
[0037] 1. Synthesis of STP1
[0038] The synthetic route of STP1 is as follows figure 1 shown;
[0039] 1) Synthesis of STP1-1:
[0040] At room temperature, NaH (210mmol), dimethyl carbonate (17.6mL, 210mmol), and 30mL toluene were added to a 250mL three-necked flask, and the temperature was raised to 120°C to reflux, and acetophenone (83mmol) in 30mL of toluene was added dropwise. After 30 minutes of reaction, TLC monitored the complete reaction of acetophenone, then added 100 mL of ice water, and adjusted the pH to 6-7 with 6N HCl. Separation, extraction of the aqueous phase with ether (1×100mL, 2×60mL), combined organic phases, washed once with water, washed once with saturated brine, dried over anhydrous sodium sulfate, spin-dried the solvent, purified by column chromatography (petroleum ether: ethyl acetate =20:1 elution), a white solid was obtained with a yield of 80-90%.
[0041] 2) Synthesis of STP1-2:
[0042]At room temperature, to a suspension of NaH (15 mmol)...
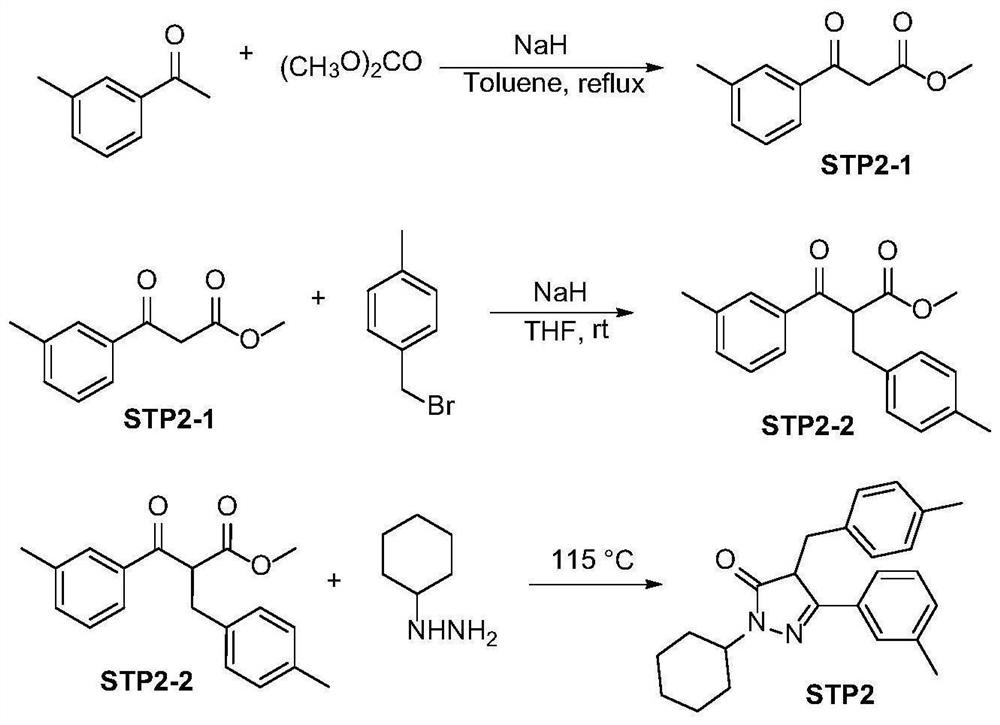
Embodiment 2
[0098] Quantitative evaluation of the inhibitory ability of 1-cyclohexylpyrazolone compounds on carboxylesterase 1 (hCES1A)
[0099] Using the hydrolytic metabolism of D-luciferin methyl ester (DME) carboxylesterase 1 as a probe reaction, bioluminescence was used to detect the effect of 1-cyclohexylpyrazolone compounds on carboxylic acid with the help of human liver microsome in vitro incubation system IC of esterase 1 (hCES1A) inhibition 50 :
[0100] a) 100 microliters of in vitro metabolic reaction system contains phosphate buffer solution (0.1M PBS, 94 μL) with a pH of 6.5, the final concentration of human liver microsomal protein is 2 μg / mL (HLM, 2 μL), and the final concentration range of inhibitors is 0.1μM-80μM (2μL), shake pre-incubation on a microplate reader for 10 minutes at 37°C;
[0101] b) Add D-fluorescein methyl ester (DME) substrate (2 μL, final concentration 3 μM) to the reaction system to initiate the reaction, and incubate the reaction with shaking on a ...
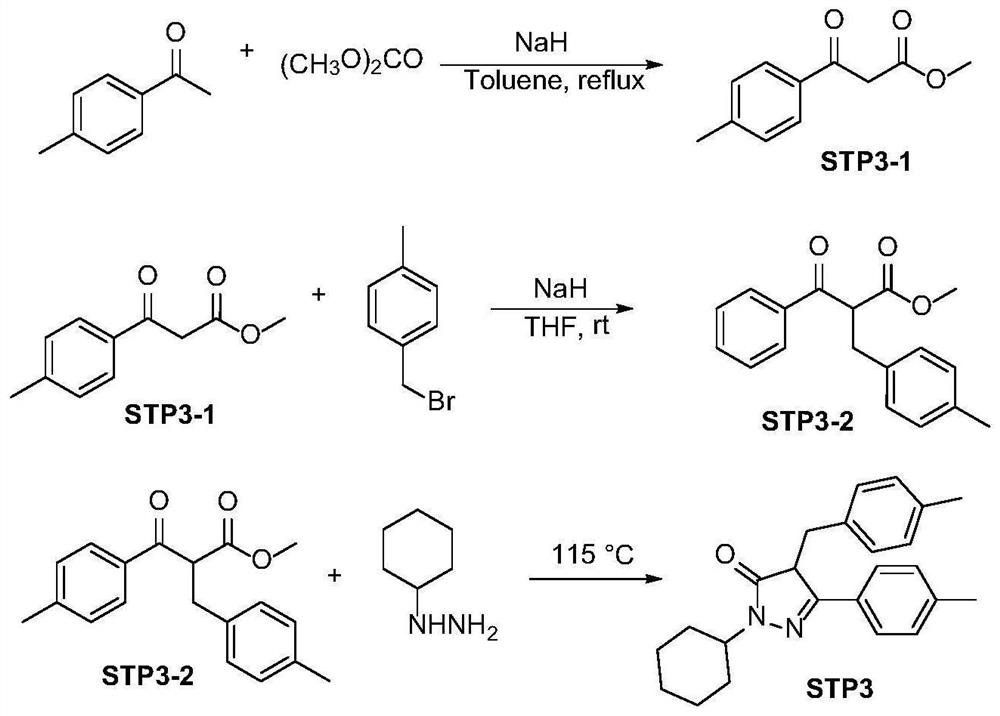
Embodiment 3
[0104] Quantitative evaluation of the inhibitory ability of 1-cyclohexylpyrazolone compounds on carboxylesterase 2 (hCES2A)
[0105] Using the hydrolytic metabolism of fluorescein diacetate FD (Fluorescein diacetate) as a probe reaction, the effect of 1-cyclohexylpyrazolone compounds on carboxylesterase 2 was determined by means of the in vitro incubation system of human liver microsomes. (hCES1A) inhibited IC 50 :
[0106] a) 200 microliters of in vitro metabolic reaction system containing phosphate buffer solution (0.1M PBS, 194 μL) with a pH of 7.4, the final concentration of human liver microsomal protein is 2 μg / mL (2 μL), and the final concentration range of inhibitors is 0.5 μM -100μM (2μL), shake pre-incubation at 37°C for 5 minutes;
[0107] b) Add FD substrate (2 μL, final concentration 5 μM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μL of acetonitrile and shake vigorously to terminate the reaction;
[0108] c)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com